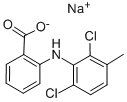
MECLOFENAMATE SODIUM
Synonym(s):2-[(2,6-Dichloro-3-methylphenyl)amino]benzoic acid sodium salt;Meclofenamic acid sodium salt
- CAS NO.:6385-02-0
- Empirical Formula: C14H10Cl2NO2.Na
- Molecular Weight: 318.13
- MDL number: MFCD00941475
- EINECS: 228-983-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-16 15:32:52
What is MECLOFENAMATE SODIUM?
Description
Meclofenamate is a time-
Originator
Meclomen,Warner Lambert,US,1980
The Uses of MECLOFENAMATE SODIUM
Meclofenamate Sodium is an NSAID used in the treatment of chronic pain.
The Uses of MECLOFENAMATE SODIUM
antiinflammatory, antipyretic
What are the applications of Application
Meclofenamate sodium is an inhibitor of 5-LO, Cox-1, and Cox-2
Definition
ChEBI: An organic sodium salt derived from meclofenamic acid. Its monohydrate is used for the treatment of dysmenorrhoea (painful periods), osteoarthritis and rheumatoid arthritis.
Indications
Meclofenamate sodium (Meclomen) is prescribed for rheumatoid arthritis and osteoarthritis. The fenamates show no clear superiority in antiinflammatory activity and may produce more adverse effects than other NSAIDs.
Manufacturing Process
A mixture consisting of 22.7 g potassium o-bromobenzoate, 16.6 g 2,6- dichloro-3-methylaniline, 12 ml N-ethylmorpholine, 60 ml diethylene glycol dimethyl ether, and 1.0 g anhydrous cupric bromide is heated in a nitrogen atmosphere at 145°C to 155°C for 2 hours. The reaction mixture is diluted with 60 ml diethylene glycol dimethyl ether and acidified with 25 ml concentrated hydrochloric acid. The acidic mixture is diluted with 100 ml of water and the liquid phase decanted from the insoluble oil. The insoluble oil is stirred with methanol and the crystalline N-(2,6-dichloro-3-methylphenyl) anthranilic acid which separates is collected and washed with methanol. The product, after recrystallization from acetone-water mixture, melts at 248°C to 250°C.
brand name
Meclomen (Parke-Davis).
Therapeutic Function
Antiinflammatory
General Description
Meclofenamate sodium (Meclomen) is available for use inthe treatment of acute and chronic RA, OA, and primarydysmenorrhea. It is metabolized in a similar manner tomefenamic acid discussed above, thus a similar restriction isalso applied to meclofenamate. The most significant side effectsare GI, including diarrhea.
Pharmacokinetics
Meclofenamate sodium is rapidly and almost completely absorbed following oral administration, reaching peak plasma
levels within 2 hours. It is highly bound to plasma proteins (99%) and has a plasma half-life of 2 to 4 hours.
Metabolism involves oxidation of the methyl group, aromatic hydroxylation, monodehalogenation, and conjugation.
Urinary excretion accounts for approximately 75% of the administered dose. The major metabolite is the product of
3′-methyl oxidation and has been shown to possess anti-inflammatory activity.
Clinical Use
Meclofenamate sodium is indicated for the relief of mild to moderate pain, the acute and chronic treatment of rheumatoid arthritis and osteoarthritis, the treatment of primary dysmenorrhea, and the treatment of idiopathic, heavy menstrual blood loss.
Properties of MECLOFENAMATE SODIUM
| Melting point: | 287-291 °C |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | White solid. |
| color | White to Off-White |
| Water Solubility | Soluble in water at 50mg/ml. Also soluble in DMF, DMSO or ethanol |
| Merck | 14,5779 |
| Stability: | Hygroscopic |
Safety information for MECLOFENAMATE SODIUM
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for MECLOFENAMATE SODIUM
MECLOFENAMATE SODIUM manufacturer
Aurazia Chem Laboratories Private Limited
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![2-[(2-Chlorophenyl)amino]-benzaldehyde](https://img.chemicalbook.in/CAS/GIF/71758-44-6.gif)

You may like
-
 6385-02-0 Meclofenamate sodium 98%View Details
6385-02-0 Meclofenamate sodium 98%View Details
6385-02-0 -
 Sodium Meclofenamate CAS 6385-02-0View Details
Sodium Meclofenamate CAS 6385-02-0View Details
6385-02-0 -
 Meclofenamate Sodium CAS 6385-02-0View Details
Meclofenamate Sodium CAS 6385-02-0View Details
6385-02-0 -
 Meclofenamic acid sodium salt CAS 6385-02-0View Details
Meclofenamic acid sodium salt CAS 6385-02-0View Details
6385-02-0 -
 Meclofenamate Sodium CAS 6385-02-0View Details
Meclofenamate Sodium CAS 6385-02-0View Details
6385-02-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1