Magnesium fluosilicate
- CAS NO.:16949-65-8
- Empirical Formula: F6MgSi
- Molecular Weight: 166.38
- MDL number: MFCD00016196
- EINECS: 241-022-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:52
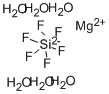
What is Magnesium fluosilicate?
Description
Magnesium silicofluoride is a white crystallinesolid. Molecular weight= 274.52. Hazard Identification(based on NFPA-704 M Rating System): Health 3,Flammability 0, Reactivity 0. Soluble in water.
General Description
Crystalline solid. Decomposes at 120°C. Contact may cause slight irritation to skin, eyes, and mucous membranes. May be slightly toxic by ingestion. Used to make other chemicals.
Air & Water Reactions
Water soluble.
Reactivity Profile
Magnesium fluosilicate gives basic aqueous solutions. Reacts with acids. Does not usually react as either oxidizing agents or reducing agents.
Health Hazard
Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Containers may explode when heated. Runoff may pollute waterways.
Flammability and Explosibility
Non flammable
Safety Profile
Moderately toxic by ingestion. When heated to decomposition it emits toxic vapors of magnesium and F-.
Potential Exposure
Magnesium silicofluoride is used as aconcrete hardener and waterproofing agent. Hexafluoro isused as an agricultural chemical.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area away from strong acids (such as hydrochloric, sulfuric, and nitric).
Shipping
This compound requires a shipping label of“POISONOUS/TOXIC MATERIALS.” It falls in HazardClass 6.1 and Packing Group III.
Incompatibilities
Strong acids.
Properties of Magnesium fluosilicate
| Melting point: | 120°C |
| Boiling point: | >120°C |
| Density | 1.788 |
| vapor pressure | 0.001Pa at 25℃ |
| form | Powder |
| Specific Gravity | 1.788 |
| Water Solubility | 498.4g/L at 18.3℃ |
| Hydrolytic Sensitivity | 2: reacts with aqueous acid |
| CAS DataBase Reference | 16949-65-8(CAS DataBase Reference) |
| EPA Substance Registry System | Magnesium fluosilicate (16949-65-8) |
Safety information for Magnesium fluosilicate
Computed Descriptors for Magnesium fluosilicate
Magnesium fluosilicate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 16949-65-8 Magnesium hexafluorosilicate 98%View Details
16949-65-8 Magnesium hexafluorosilicate 98%View Details
16949-65-8 -
 16949-65-8 98%View Details
16949-65-8 98%View Details
16949-65-8 -
 Magnesium Fluosilicate CAS: 16949-65-8, PowderView Details
Magnesium Fluosilicate CAS: 16949-65-8, PowderView Details
16949-65-8 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
