Luminol
Synonym(s):3-Aminophthalhydrazide;5-Amino-2,3-dihydro-1,4-phthalazinedione;5-Amino-2,3-dihydrophthalazine-1,4-dione;Luminol
- CAS NO.:521-31-3
- Empirical Formula: C8H7N3O2
- Molecular Weight: 177.16
- MDL number: MFCD00006890
- EINECS: 208-309-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-24 18:12:00
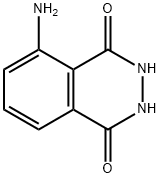
What is Luminol?
Chemical properties
yellow crystals or beige powder
The Uses of Luminol
Luminol is a chemiluminescent probe that has been used to detect myeloperoxidase-mediated oxidative events in granulocytes and for chemiluminescence analysis of metal cations and blood.
The Uses of Luminol
Detection of copper, iron, peroxides, cyanides.
The Uses of Luminol
Luminol is used in the detection of copper, iron, peroxides and cyanides. It exhibits chemiluminescence and utilized to measure opsonic and phagocytic function. It acts as a biological sensor and involved in the detection of polymorphonuclear leukocytes response in a patient with a myeloperoxidase deficiency. Further, it is used as a forensic test for blood.
What are the applications of Application
Luminol is a chemiluminescence generating reagent for biological and metal cation assays
General Description
Yellow crystals or light beige powder.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Oxidation of 3-Aminophthalhydrazide is accompanied by a striking emission of light. . .
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition 3-Aminophthalhydrazide emits toxic fumes.
Fire Hazard
Flash point data for 3-Aminophthalhydrazide are not available, but 3-Aminophthalhydrazide is probably combustible.
Synthesis
The synthesis of Luminol is as follows: To a reaction tube, add 140 mg of 3-nitrophthalhydrazide and 1.0 mL of 3 M sodium hydroxide solution. Stir with a rod, and to the resulting deep brown-red solution add 0.6 g of sodium hydrosulfite dihydrate (Na2S2O4 · 2 H2O, MW 210.2). Wash down the sides of the tube with a small amount of water. Heat to a gentle boil and keep the tube hot for 5 minutes. During this time some product may begin to crystallize. Add 0.4 mL of acetic acid, cool the tube in cold water, and stir. Collect the light-yellow product Luminol by suction filtration.
Storage
Room temperature
Purification Methods
Dissolve luminol in KOH solution, treat with Norit (charcoal), filter and precipitate it with conc HCl. [Hardy et al. Talanta 24 297 1977.] Store it in the dark in an inert atmosphere, because its structure changes during its luminescence. It has been recrystallised from 0.1M KOH [Merenyi et al. J Am Chem Soc 108 77716 1986]. [Beilstein 25 II 389, 25 III/IV 4192.]
Properties of Luminol
| Melting point: | >300 °C (lit.) |
| Boiling point: | 309.07°C (rough estimate) |
| Density | 1.3393 (rough estimate) |
| refractive index | 1.6500 (estimate) |
| storage temp. | Store below +30°C. |
| solubility | Solubility Insoluble in water, soluble in dimethyl sulfoxide, base |
| form | Powder |
| pka | (Calcd.) 10.50 ± 0.20;;0.58 ± 0.20(at 25℃) |
| color | Dark green to dark blue-purple |
| PH Range | NonH uorescence (6.0) to blue H uorescence (7.0) |
| Water Solubility | <0.1 g/100 mL at 19 ºC |
| λmax | 425nm |
| Merck | 14,5600 |
| BRN | 383929 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, strong acids, strong bases, strong reducing agents. Emits light on reaction with oxidizers. |
| Major Application | Night vision devices, identification of product forgeries, determination of nitride in food, analytical chemistry, pharmaceuticals, iodine in biological materials, evaluating phagocytes, detecting tumors, DNA, biosensors, assay of fungi, bacteria, yeasts, assay of lipase activity |
| CAS DataBase Reference | 521-31-3(CAS DataBase Reference) |
| NIST Chemistry Reference | 3-Aminophthalhydrazide(521-31-3) |
| EPA Substance Registry System | Luminol (521-31-3) |
Safety information for Luminol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Luminol
| InChIKey | HWYHZTIRURJOHG-UHFFFAOYSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![5-[(1-cyclopropylethyl)amino]-1,2,3,4-tetrahydrophthalazine-1,4-dione](https://img.chemicalbook.in/CAS/20200119/GIF/CB41769704.gif)
![5-[(3-methylbutyl)amino]-1,2,3,4-tetrahydrophthalazine-1,4-dione](https://img.chemicalbook.in/CAS/20200401/GIF/CB61770531.gif)
You may like
-
 521-31-3 Luminol 99%View Details
521-31-3 Luminol 99%View Details
521-31-3 -
 Luminol extrapure CAS 521-31-3View Details
Luminol extrapure CAS 521-31-3View Details
521-31-3 -
 Luminol, for biochemistry CAS 521-31-3View Details
Luminol, for biochemistry CAS 521-31-3View Details
521-31-3 -
 Luminol, for chemiluminescene CAS 521-31-3View Details
Luminol, for chemiluminescene CAS 521-31-3View Details
521-31-3 -
![Luminol [Chemiluminescence Reagent] CAS 521-31-3](https://img.chemicalbook.in//Content/image/CP5.jpg) Luminol [Chemiluminescence Reagent] CAS 521-31-3View Details
Luminol [Chemiluminescence Reagent] CAS 521-31-3View Details
521-31-3 -
 Luminol CAS 521-31-3View Details
Luminol CAS 521-31-3View Details
521-31-3 -
 Luminol 98% (HPLC) CAS 521-31-3View Details
Luminol 98% (HPLC) CAS 521-31-3View Details
521-31-3 -
 LUMINOL For Synthesis CAS 521-31-3View Details
LUMINOL For Synthesis CAS 521-31-3View Details
521-31-3
