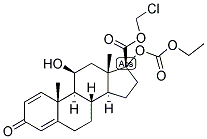
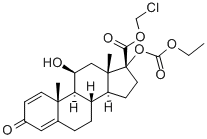
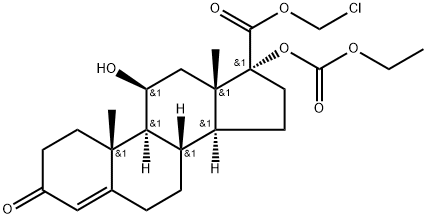
Loteprednol etabonate
Synonym(s):(11b,17a)-17-[(Ethoxycarbonyl)oxy]-11-hydroxy-3-oxo-Androsta-1,4-diene-17-carboxylic acid chloromethyl ester;CDDD 5604;HGP 1;P 5604
- CAS NO.:82034-46-6
- Empirical Formula: C24H31ClO7
- Molecular Weight: 466.95
- MDL number: MFCD00870765
- EINECS: 200-010-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
What is Loteprednol etabonate?
Absorption
Loteprednol etabonate (LE) demonstrates good ocular permeation properties as it is lipid soluble, allowing the agent to penetrate into cells with relative ease .
Results from the ocular administration of loteprednol in normal, healthy volunteers have shown that there are low or undetectable concentrations of either unchanged material or its metabolite . Following twice-daily unilateral topical ocular dosing of LE for 14 days in healthy subjects, the plasma concentrations of loteprednol etabonate were below the limit of quantitation (1 ng/mL) at all time points . These finds suggest that limited, if any, systemic absorption of LE occurs .
Toxicity
The most common adverse drug reactions reported during clinical trials for the medication were eye pain and posterior capsular opacification, both of which may also be the consequence of the very surgical procedures performed on the eye(s) .
The agent is not absorbed systemically following topical ophthalmic administration and maternal use is not expected to result in fetal exposure to the drug .
The medication is not absorbed systemically by the mother following topical ophthalmic administration, and breastfeeding is not expected to result in exposure of the child to the agent .
Long-term animal studies have not been conducted to evaluate the carcinogenic potential of loteprednol etabonate. Loteprednol etabonate was not genotoxic in vitro in the Ames test, the mouse lymphoma thymidine kinase (tk) assay, or in a chromosome aberration test in human lymphocytes, or in vivo in the single dose mouse micronucleus assay .
Overdose is not expected to be likely to occur after ocular administration .
Description
Loteprednol etabonate was introduced in the US as Lotemax (opththalmic suspension at 0.5%) for the treatment of steroid-responsive inflammatory conditions of the palpebral and bulbar conjunctiva, cornea and anterior segment of the ocular globe, and as Alrex (opththalmic suspension at 0.2%) for the symptomatic treatment of seasonal allergic conjunctivitis. Loteprednol etabonate is a novel soft corticosteroid with a superior efficacy and an improved safety profile compared to prior ophthalmic steroids due to its metabolic lability and a fast enzymatic transformation to inactive metabolite. A combination of Lotemax with the antibiotic Tobramycin is currently under development.
Chemical properties
x
Originator
Pharmos (US)
The Uses of Loteprednol etabonate
Biological Activity Chemical Information Tech Support & FAQs Biological Activity Loteprednol etabonate is an anti-inflammatory corticosteroid used in ophthalmology. It is used for the treatment of steroid responsive inflammatory conditions of the eye su
The Uses of Loteprednol etabonate
An ophthalmic corticosteroid. Used as an anti-inflammatory.
Background
Loteprednol Etabonate (LE) is a topical corticoid anti-inflammatory. It is used in ophthalmic solution for the treatment of steroid responsive inflammatory conditions of the eye such as allergic conjunctivitis, uveitis, acne rosacea, superficial punctate keratitis, herpes zoster keratitis, iritis, cyclitis, and selected infective conjunctivitides. As a nasal spray, it can be used for the treatment and management of seasonal allergic rhinitis.
Most prescription LE products, however, tend to be indicated for the treatment of post-operative inflammation and pain following ocular surgery . A number of such new formulations that have been approved include Kala Pharmaceutical's Inveltys - the first twice-daily (BID) ocular corticosteroid approved for this indication, designed specifically to enhance patient compliance and simplified dosing compared to all other similar ocular steroids that are dosed four times daily .
Moreover, LE was purposefully engineered to be a 'soft drug', one that is designed to be active locally at the site of administration and then rapidly metabolized to inactive components after eliciting its actions at the desired location, thereby subsequently minimizing the chance for adverse effects .
Indications
A number of prescription loteprednol etabonate ophthalmic products are specifically indicated for the treatment of post-operative inflammation and pain following ocular surgery .
What are the applications of Application
Loteprednol Etabonate is an ophthalmic corticosteroid
Definition
ChEBI: Loteprednol etabonate is an etabonate ester, an 11beta-hydroxy steroid, a steroid ester, an organochlorine compound, a steroid acid ester and a 3-oxo-Delta(1),Delta(4)-steroid. It has a role as an anti-inflammatory drug. It is functionally related to a loteprednol.
Manufacturing Process
To a solution of hydrocortisone (15 g, 0.04 mol) in 120 ml of THF and 30 ml of methanol at room temperature is added a warm solution of sodium metaperiodate (25.7 g, 0.12 mol) in 100 ml of water. The reaction mixture is stirred at room temperature for 2 hours, then is concentrated under reduced pressure to remove the tetrahydrofuran and methanol. The solid is triturated with 50 ml of water, separated by filtration, washed with water and dried in vacuo at 50°C for 3 hours. The product, 11β,17α-dihydroxyandrost-4-en-3- one-17β-carboxylic acid (i.e., cortienic acid), is obtained in approximately 96% yield (13.76 g); melting point 231-234°C.
To a cold solution of 11β,17α-dihydroxyandrost-4-en-3-one-17β-carboxylic acid
(5% weight/volume; 1 mol) and triethylamine (4 mol) in dichloromethane is
added a 50% (weight/volume) solution of ethyl chloroformate (3.9 mol) in
dichloromethane. The reaction mixture is allowed to warm to room
temperature over a 2 hour period. The triethylamine hydrochloride precipitate
which forms is removed by filtration and the filtration is washed successively
with 3% sodium bicarbonate, 1% hydrochloric acid and water. The organic
layer is separated, dried with magnesium sulfate, and filtered. The filtrate is
concentrated in vacuo to a foam.
The foam is used in the next step below or chromatographed and crystallized
for analysis. The product 17α-ethoxycarbonyloxy-11β-hydroxyandrost-4-en-3-
one-17β-carboxylic acid, melting at 192-195°C C after chromatography and
crystallization.
17α-Ethoxycarbonyloxy-11β-hydroxyandrost-4-en-3-one-17β-carboxylic acid is
combined with an equivalent amount of 1 N sodium hydroxide in methanol
and that solution is diluted to 100 times the original volume with ethyl ether.
The suspension which results is refrigerated for 1 hour. Then, the crystals
which form are removed by filtration, dried in an evacuated desiccator, and
dissolved in hexamethylphosphoramide (10% weight/volume). A portion of the
resultant solution containing 1 mole of the acid salt, i.e. of sodium 17αethoxycarbonyloxy-11β-hydroxyandrost-4-en-3-one-17β-carboxylate, is
combined with 4 moles of chloromethyl iodide. The reaction mixture is
maintained at room temperature for 3 hours, then is diluted to 10 times the
original volume with ethyl acetate. The diluted reaction mixture is washed
successively with 5% sodium thiosulfate, 3% sodium bicarbonate, and water.
The organic layer is separated, dried with magnesium sulfate and filtered. The
filtrate is concentrated in vacuo to a foam. The foam is purified by
crystallization from ethyl ether or tetrahydrofuran/hexane. There is thus
obtained chloromethyl-17α-ethoxycarbonyloxy-11β-hydroxyandrost-4-en-3-
one-17β-carboxylate, melting at 197-200°C after crystallization.
brand name
Alrex (Bausch & Lomb); Lotemax (Bausch & Lomb); Lotemax (Pharmos);Lotemax (0.5%).
Therapeutic Function
Glucocorticoid
General Description
Loteprednol etabonate,chloromethyl 17α-[(ethoxycarbonyl)oxy]-11β-hydroxy-3-oxoandrosta-1,4-diene-17-carboxylate (Alrex,Lotemax), has a modified carboxylate at the C17 positionrather than the typical ketone functionality. This modificationmaintains affinity for the GR but allows facile metabolismto inactive metabolites. This limits the systemic actionof the drug. Loteprednol etabonate is used as anophthalmic suspension that has greatly reduced systemicaction because of rapid metabolism to the inactive carboxylate.
Biochem/physiol Actions
Loteprednol Etabonate is an anti-inflammatory corticosteroid (ophthalmology).
Pharmacokinetics
Loteprednol etabonate (LE) belongs to a unique class of corticosteroids with potent anti-inflammatory effects designed to be active at the site of action . Animal studies have shown that LE has a binding affinity to steroid receptors that is 4.3 times greater than dexamethasone . This particular class of steroids consists of bioactive molecules whose in-vivo transformation to non-toxic substances can be predicted from their chemistry and knowledge of enzymatic pathways in the body . Cortienic acid is an inactive metabolite of hydrocortisone and analogs of cortienic acid are also devoid of corticosteroid activity . Specifically, LE is an ester derivative of one of these analogs, cortienic acid etabonate . In particular, LE possesses a metabolically labile 17 beta-chloromethyl ester function which was designed in order to be hydrolyzed to an inactive carboxylic acid moiety . This inactive metabolite is more hydrophilic and is thus readily eliminated from the body . LE also exhibits good ocular permeation properties and good skin permeation properties .
Metabolism
Loteprednol etabonate (LE) is readily and extensively metabolized to two inactive metabolites, PJ-90 (Δ1-cortienic acid) and PJ-91 (Δ1-cortienic acid etabonate) . Metabolism occurs locally in ocular tissues, and to the extent that loteprednol etabonate reaches the systemic circulation, likely the liver and other tissues into which it distributes .
In particular, studies have demonstrated that LE (chloromethyl 17alpha-ethoxycarbonyloxy-11beta-hydroxy-3-oxoandrosta-1,4-diene) is rapidly hydrolyzed at the location of its 17beta-chloromethyl ester function by paraoxonase 1 in human plasma at the site of administration at the level of the affected eye tissue to the 17beta-carboxylate PJ-91 metabolite and PJ-90 metabolite . Both metabolites are considered inactive .
Properties of Loteprednol etabonate
| Melting point: | 220.5-223.5 |
| Boiling point: | 600.1±55.0 °C(Predicted) |
| Density | 1.31±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: soluble5mg/mL, clear (warmed) |
| form | powder |
| pka | 14.06±0.70(Predicted) |
| color | white to beige |
| Water Solubility | <1mg/L(23 ºC) |
| Stability: | Hygroscopic |
Safety information for Loteprednol etabonate
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H361:Reproductive toxicity H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. |
Computed Descriptors for Loteprednol etabonate
| InChIKey | DMKSVUSAATWOCU-ICASLIHPSA-N |
Loteprednol etabonate manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

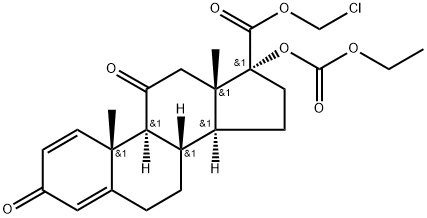
![Androsta-1,4-diene-17-carboxylic acid, 17-[(ethoxycarbonyl)oxy]-11-hydroxy-3-oxo-, (11β,17α)-](https://img.chemicalbook.in/CAS/20180808/GIF/133991-63-6.gif)

You may like
-
 Loteprednol Etabonate 99%View Details
Loteprednol Etabonate 99%View Details -
 loteprednol etabonate 82034-46-6 98%View Details
loteprednol etabonate 82034-46-6 98%View Details
82034-46-6 -
 82034-46-6 loteprednol etabonate 98%View Details
82034-46-6 loteprednol etabonate 98%View Details
82034-46-6 -
 loteprednol etabonate 98%View Details
loteprednol etabonate 98%View Details
82034-46-6 -
 loteprednol etabonate 82034-46-6 98%View Details
loteprednol etabonate 82034-46-6 98%View Details
82034-46-6 -
 Loteprednol Etabonate CAS 82034-46-6View Details
Loteprednol Etabonate CAS 82034-46-6View Details
82034-46-6 -
 Loteprednol etabonate 98% (HPLC) CAS 82034-46-6View Details
Loteprednol etabonate 98% (HPLC) CAS 82034-46-6View Details
82034-46-6 -
 Loteprednol Etabonate CAS 82034-46-6View Details
Loteprednol Etabonate CAS 82034-46-6View Details
82034-46-6
