Lithium perchlorate trihydrate
- CAS NO.:13453-78-6
- Empirical Formula: ClH4LiO5
- Molecular Weight: 126.42
- MDL number: MFCD00149765
- EINECS: 680-433-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-10-17 17:11:56

What is Lithium perchlorate trihydrate?
Chemical properties
White crystal
The Uses of Lithium perchlorate trihydrate
Lithium perchlorate trihydrate is used as am oxidizing agent.
The Uses of Lithium perchlorate trihydrate
Lithium perchlorate trihydrate is used as an oxygen source for chemical oxygen generators as it liberates oxygen at high temperatures. Excellent solubility of lithium perchlorate permits its use in Diels-Alder reactions. It is also employed in lithium-ion batteries as an electrolyte and acts as a chaotropic agent to denature proteins. It is used in the Baylis-Hillman reaction as a catalyst in the coupling of alpha, beta-unsaturated carbonyl compounds with aldehydes.
General Description
Crystal structure of lithium perchlorate trihydrate (LiClO4.3H2O) has been investigated by X-ray and neutron diffraction studies. It exhibits pyroelectric properties.
Properties of Lithium perchlorate trihydrate
| Melting point: | 95 °C |
| Boiling point: | decomposes at 470℃ [KIR79] |
| Density | 1.841 g/mL at 25 °C(lit.) |
| solubility | very soluble in H2O, ethanol, acetone;insoluble in ethyl ether |
| form | Crystals and/or Chunks |
| Specific Gravity | 1.841 |
| color | White |
| Odor | Odorless |
| Water Solubility | 597 g/L (25 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,5539 |
| Stability: | hygroscopic |
| CAS DataBase Reference | 13453-78-6(CAS DataBase Reference) |
Safety information for Lithium perchlorate trihydrate
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Lithium perchlorate trihydrate
Abamectin manufacturer
Pallav Chemicals And Solvents Pvt Ltd
1Y
Phone:+91-9136093115
Whatsapp: +91- 9136093115
product: 13453-78-6 Lithium Perchlorate Trihydrate 99% Extrapure 99%
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran




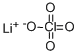

You may like
-
 Lithium perchlorate trihydrate CAS 13453-78-6View Details
Lithium perchlorate trihydrate CAS 13453-78-6View Details
13453-78-6 -
 Lithium perchlorate trihydrate CAS 13453-78-6View Details
Lithium perchlorate trihydrate CAS 13453-78-6View Details
13453-78-6 -
 13453-78-6 Lithium Perchlorate Trihydrate 99% Extrapure 99%View Details
13453-78-6 Lithium Perchlorate Trihydrate 99% Extrapure 99%View Details
13453-78-6 -
 Lithium perchlorate, 99% CAS 13453-78-6View Details
Lithium perchlorate, 99% CAS 13453-78-6View Details
13453-78-6 -
 LITHIUM PERCHLORATE TRIHYDRATE Extra Pure CAS 13453-78-6View Details
LITHIUM PERCHLORATE TRIHYDRATE Extra Pure CAS 13453-78-6View Details
13453-78-6 -
 Lithium perchlorate trihydrate 99% CAS 13453-78-6View Details
Lithium perchlorate trihydrate 99% CAS 13453-78-6View Details
13453-78-6 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4
Statement: All products displayed on this website are only used for non medical purposes such as industrial applications or scientific research, and cannot be used for clinical diagnosis or treatment of humans or animals. They are not medicinal or edible.