LITHIUM NITRIDE
Synonym(s):Trilithium nitride
- CAS NO.:26134-62-3
- Empirical Formula: Li3N
- Molecular Weight: 34.83
- MDL number: MFCD00016186
- EINECS: 247-475-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
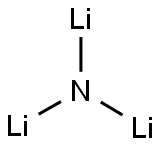
What is LITHIUM NITRIDE?
Chemical properties
reddish brown crystal(s) or freely flowing powder(s); slowly decomposed by atmospheric moisture; ruby red; hexagonal, a=0.3658 nm, c=0.3882nm; conductivity, 227°C, 0.04 (ohm· cm)?1; one of most effective solid ionic conductors; can be prepared by direct reaction of Li and nitrogen; used as a nitriding agent in metallurgy [HAW93] [STR93] [CIC73] [KIR81]
The Uses of LITHIUM NITRIDE
Lithium nitride is used in metallurgy and chemical synthesis. It is also used to store hydrogen and acts as a source of the nitride ion. It is involved in the preparation of lithium hydride and lithium amide as well as a reducing agent.
Preparation
Lithium nitride is prepared by the reaction of nitrogen gas with lithium metal. The reaction may be carried out at temperatures well above the melting point of lithium metal or using solid lithium metal at temperatures even below 100°C. Lithium nitride, a red crystalline solid, reacts with water to yield lithium hydroxide and ammonia. It is ultimately converted to lithium carbonate in the air. The compound readily reacts with water and carbon dioxide. It is also flammable, particularly when it is finely divided. For these reasons lithium nitride is stored and handled under an inert atmosphere.
Definition
ChEBI: Lithium nitride is a lithium salt and a nitride.
General Description
A reddish brown powder. Insoluble in most organic solvents. Used in metallurgy and chemical synthesis.
Reactivity Profile
LITHIUM NITRIDE is a strongly basic reducing agent. Incompatible with oxidizing agents such as atmospheric oxygen. Violently incompatible with acids, particularly oxidizing acids. Reacts violently with copper(I) chloride to produce metallic copper [Mellor, 1940, vol.8, 100]. Reacts exothermically with silicon tetrafluoride, leading to explosion [Chem. Brit., 1979, 15, 282-283].
Health Hazard
Inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solutions on contact with water. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
Fire Hazard
Produce flammable gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Runoff may create fire or explosion hazard.
Flammability and Explosibility
Pyrophoric
Safety Profile
A powerful reducing agent. Upon contact with moisture, it decomposes into lithmm hydroxide, lithium compounds, and ammonia. The powder may ignite spontaneously in moist air. Flammable at elevated temperatures; ignites and burns intensely in air. Violent reaction with dicon tetrafluoride, copperp) chloride + heat. To fight fire, use dry chemical, sand, graphite; avoid use of water or carbon tetrachloride. When heated to decomposition it emits very toxic fumes of Liz0 and NOx. Used as a strong reducing agent in organic synthesis and a solid electrolyte in lithium batteries. See also LITHIUM COMPOUNDS and NITRIDES.
Properties of LITHIUM NITRIDE
| Melting point: | 845°C |
| Density | 1.3 g/mL at 25 °C(lit.) |
| solubility | Insoluble in organic solvents. |
| form | Powder |
| Specific Gravity | 1.38 |
| color | reddish-brown |
| Water Solubility | reacts with H2O, yielding LiOH and ammonia; insoluble polyethers [HAW93] |
| Sensitive | Moisture Sensitive |
| CAS DataBase Reference | 26134-62-3(CAS DataBase Reference) |
| EPA Substance Registry System | Lithium nitride (Li3N) (26134-62-3) |
Safety information for LITHIUM NITRIDE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H260:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P223:Keep away from any possible contact with water, because of violent reaction and possible flash fire. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for LITHIUM NITRIDE
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Lithium nitride CAS 26134-62-3View Details
Lithium nitride CAS 26134-62-3View Details
26134-62-3 -
 Lithium nitride CAS 26134-62-3View Details
Lithium nitride CAS 26134-62-3View Details
26134-62-3 -
 Lithium nitride, ≥99.5% CAS 26134-62-3View Details
Lithium nitride, ≥99.5% CAS 26134-62-3View Details
26134-62-3 -
 Lithium nitride CAS 26134-62-3View Details
Lithium nitride CAS 26134-62-3View Details
26134-62-3 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
