LITHIUM METABORATE
Synonym(s):Boric acid lithium salt
- CAS NO.:13453-69-5
- Empirical Formula: BH2LiO2
- Molecular Weight: 51.76
- MDL number: MFCD00011089
- EINECS: 236-631-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
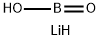
What is LITHIUM METABORATE?
Chemical properties
White powder
The Uses of LITHIUM METABORATE
Lithium metaborate is used for flux.
The Uses of LITHIUM METABORATE
Lithium Metaborate is commonly used as a fused compound or a component for an assay. It has been used for the analysis of wild rice for silicon, calcium, magnesium and potassium. Lithium metaborate was used to fuse with solid samples for decomposition.
What are the applications of Application
Lithium Metaborate is a standard component used for assays
Preparation
Lithium metaborate, LiBO2, may be prepared by fusing together either lithium hydroxide or lithium carbonate and boric acid in the proper stoichiometric ratio. Recrystallization from water at about room temperature yields lithium metaborate- octahydrate, LiBO2·8H2O. The octahydrate may be dried to yield a dihydrate, LiBO2-2H2O, or a half-hydrate, LiBO2·0.5H2O.
General Description
Lithium metaborate (LiBO2) is the lithium salt of boric acid. It can be synthesized by reacting orthoboric acid with lithium carbonate. Its crystals belong to the monoclinic crystal system having space group P21/c. Three polymorphic forms have been identified on ambient pressure devitrification of LiBO2: α-, γ- and β′-LiBO2.
Flammability and Explosibility
Not classified
Structure and conformation
Lithium metaborate has several crystal forms.
Below 100 °C, LiBO2·8H2O is firstly dehydrated to a lower hydrate LiBO2·2H2O [1].
When the temperature is higher than 190°C, the dehydrated amorphous hydrate could crystallize into other poorly crystalline phases designated as LiBO2·xH2O (x=0.3/0.5)[2].
An anhydrous monoclinic α-LiBO2 could be obtained when the baking temperature reaches 600 °C. The α form consists of infinite chains of trigonal planar metaborate anions [BO2O-]n.
At 950 °C, by applying 15 kbar of pressure to a LiCl flux containing α-LiBO2, α-LiBO2 could be converted to metastable γ-LiBO2 crystals which were small, but of high quality[3]. γ-LiBO2 has tetragonal symmetry with lattice parameter a=4.1961 ?, c=6.5112 ?, space group I-42d and a density of 2.882 g/cm3.
References
[1] E.I.Kamitsos, A.P.Patsis, M.A.Karakassides, G.D.Chryssikos, Infrared reflectance spectra of lithium borate glasses, Journal of Non-Crystalline Solids, 1990, 126, 52-67.
[2] Li Lei, Duanwei He, Kai He, Jiaqian Qin, Shanmin Wang, Pressure-induced coordination changes in LiBO2, Journal of Solid State Chemistry, 2009, 182, 3041-3048.
[3] Colin D. McMillen, Henry G. Giesber, Joseph W. Kolis, The hydrothermal synthesis, growth, and optical properties of γ-LiBO2, Journal of Crystal Growth, 310, 2008, 299-305.
Properties of LITHIUM METABORATE
| Melting point: | 845 °C (lit.) |
| Density | 1,4 g/cm3 |
| storage temp. | Store at +5°C to +30°C. |
| solubility | soluble in ethanol. |
| form | Powder |
| color | White |
| Specific Gravity | 1.4 |
| Water Solubility | Soluble in water(27.5g/L). |
| Sensitive | Hygroscopic |
| Exposure limits | ACGIH: TWA 2 mg/m3; STEL 6 mg/m3 |
| Stability: | Stable. |
| CAS DataBase Reference | 13453-69-5(CAS DataBase Reference) |
| EPA Substance Registry System | Lithium metaborate (13453-69-5) |
Safety information for LITHIUM METABORATE
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for LITHIUM METABORATE
LITHIUM METABORATE manufacturer
Parad Corporation Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Lithium metaborate, Anhydrous CAS 13453-69-5View Details
Lithium metaborate, Anhydrous CAS 13453-69-5View Details
13453-69-5 -
 Lithium metaborate, Anhydrous CAS 13453-69-5View Details
Lithium metaborate, Anhydrous CAS 13453-69-5View Details
13453-69-5 -
 Lithium metaborate, anhydrous CAS 13453-69-5View Details
Lithium metaborate, anhydrous CAS 13453-69-5View Details
13453-69-5 -
 Lithium metaborate CAS 13453-69-5View Details
Lithium metaborate CAS 13453-69-5View Details
13453-69-5 -
 Lithium metaborate CAS 13453-69-5View Details
Lithium metaborate CAS 13453-69-5View Details
13453-69-5 -
 Lithium metaborate CAS 13453-69-5View Details
Lithium metaborate CAS 13453-69-5View Details
13453-69-5 -
 Lithium metaborate CAS 13453-69-5View Details
Lithium metaborate CAS 13453-69-5View Details
13453-69-5 -
 Lithium Metaborate CASView Details
Lithium Metaborate CASView Details