Lithium bis(trimethylsilyl)amide
Synonym(s):Hexamethyldisilazane lithium salt;LiHMDS
- CAS NO.:4039-32-1
- Empirical Formula: C6H18LiNSi2
- Molecular Weight: 167.33
- MDL number: MFCD00008261
- EINECS: 223-725-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
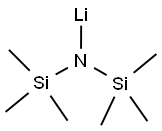
What is Lithium bis(trimethylsilyl)amide?
Description
Lithium bis(trimethylsilyl)amide (LiHMDS) is a sterically hindered strong base. It is commercially available as a solid and as a solution in a variety of solvents (ex. THF, 2Me-THF, toluene, hexane, and MTBE). Similar reagents include sodium bis(trimethylsilyl)amide (NaHMDS) and potassium bis(trimethylsilyl)amide (KHMDS).
Physical properties
distillable low-melting solid; mp 70–72 °C, bp 115 °C/1mmHg. LHMDS is a cyclic trimer in the solid state,3 whereas in benzene solution it exists in a monomer–dimer equilibrium. LHMDS exists as a tetramer-dimer mixture in hydrocarbons and as a dimer-monomer mixture in THF and ether. Treatment of LHMDS with trialkylamines increases the monomer concentration, whereas the use of diamines affords exclusively the corresponding chelated monomer. LHMDS is less soluble, less basic, more stable, and much less sensitive to air compared to lithium diisopropylamide. pKa 29.5 (THF, 27 °C).
The Uses of Lithium bis(trimethylsilyl)amide

To a suspension of methyl triphenylphosphonium bromide (2.4 g, 6.6 mmol) in THF was added tBuOK (1M in THF, 7.1 mmol) at RT. After stirring for 90 min, a solution of the SM (1.25 g, 3.35 mmol) in dry THF was added. The resulting mixture was stirred at RT for 18 h, after which time it was partitioned between saturated NH4Cl and MTBE. The org layer was dried (Na2SO4), concentrated, and purified by flash chromatography (25-30% EtOAc/hexane) to provide the product as a colorless oil. [0.3 g, 24%] [UK Pat App GB2463151A, page 141]
What are the applications of Application
Lithium bis(trimethylsilyl)amide is a base used in generating enolates
Preparation
It is conveniently prepared by the reaction of hexamethyldisilazane with n-butyllithium in hexane. For most uses the hexane is then evaporated and replaced with THF.
General Description
This product, 0.5 M in 2-methyltetrahydrofuran aligns with Safer Solvents and Auxiliaries, Use of Renewable Feedstocks and Inherently Safer Chemistry for Accident Prevention.
Flammability and Explosibility
Highly flammable
Properties of Lithium bis(trimethylsilyl)amide
| Melting point: | 73°C |
| Boiling point: | 55-56 °C |
| Density | 0.860 g/mL at 25 °C (lit.) |
| refractive index | n |
| Flash point: | 48 °F |
| storage temp. | Store below +30°C. |
| solubility | hydrolysis |
| form | Powder |
| appearance | Colorless crystalline solid |
| Specific Gravity | 0.716 |
| color | White |
| Water Solubility | hydrolysis |
| Sensitive | Air & Moisture Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| BRN | 3567910 |
| Exposure limits | ACGIH: TWA 50 ppm; STEL 100 ppm (Skin) OSHA: TWA 200 ppm(590 mg/m3) NIOSH: IDLH 2000 ppm; TWA 200 ppm(590 mg/m3); STEL 250 ppm(735 mg/m3) |
| CAS DataBase Reference | 4039-32-1(CAS DataBase Reference) |
| EPA Substance Registry System | Silanamine, 1,1,1-trimethyl-N-(trimethylsilyl)-, lithium salt (4039-32-1) |
Safety information for Lithium bis(trimethylsilyl)amide
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H228:Flammable solids H251:Self-heating substances and mixtures H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P235:Keep cool. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Lithium bis(trimethylsilyl)amide
| InChIKey | YNESATAKKCNGOF-UHFFFAOYSA-N |
Lithium bis(trimethylsilyl)amide manufacturer
JSK Chemicals
Sainor Laboratories Pvt Ltd Unit III
ASM Organics
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
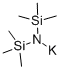
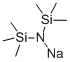

You may like
-
![Lithium bis(tri methyl silyl)amide 2.00 molar in THF [Li-HMDS] 4039-32-1 98%](https://img.chemicalbook.in//ProductImageIndia/2024-03/Raw/fe175c24-4edb-4366-9cf8-8627a046b73e.png) Lithium bis(tri methyl silyl)amide 2.00 molar in THF [Li-HMDS] 4039-32-1 98%View Details
Lithium bis(tri methyl silyl)amide 2.00 molar in THF [Li-HMDS] 4039-32-1 98%View Details
4039-32-1 -
 Lithium hexamethyldisilazide 98%View Details
Lithium hexamethyldisilazide 98%View Details -
 Lithium bis (trimethylsilyl)amide CAS 4039-32-1View Details
Lithium bis (trimethylsilyl)amide CAS 4039-32-1View Details
4039-32-1 -
 Lithium bis(trimethylsilyl)amide CAS 4039-32-1View Details
Lithium bis(trimethylsilyl)amide CAS 4039-32-1View Details
4039-32-1 -
 Lithium bis(trimethylsilyl)amide CAS 4039-32-1View Details
Lithium bis(trimethylsilyl)amide CAS 4039-32-1View Details
4039-32-1 -
 Lithium Bis(trimethylsilyl)amide (ca. 26% in Tetrahydrofuran, ca. 1.3mol/L) CAS 4039-32-1View Details
Lithium Bis(trimethylsilyl)amide (ca. 26% in Tetrahydrofuran, ca. 1.3mol/L) CAS 4039-32-1View Details
4039-32-1 -
 Lithium bis(trimethylsilyl)amide, solution 1M in THF CAS 4039-32-1View Details
Lithium bis(trimethylsilyl)amide, solution 1M in THF CAS 4039-32-1View Details
4039-32-1 -
 Lithium bis(trimethylsilyl)amide solution CAS 4039-32-1View Details
Lithium bis(trimethylsilyl)amide solution CAS 4039-32-1View Details
4039-32-1
