LINDANE
Synonym(s):δ-1,2,3,4,5,6-Hexachlorocyclohexane;δ-1,2,3,4,5,6-Hexachlorocyclohexane solution;δ-HCH solution;delta-BHC
- CAS NO.:319-86-8
- Empirical Formula: C6H6Cl6
- Molecular Weight: 290.83
- MDL number: MFCD00135945
- EINECS: 206-272-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
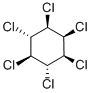
What is LINDANE?
Chemical properties
BHC is a white-to-brownish crystalline solid with a musty, phosgene-like odor.
Physical properties
Solid crystals or fine platelets with a faint musty-like odor
The Uses of LINDANE
δ-1,2,3,4,5,6-Hexachlorocyclohexane is an organochloride annd is one of the isomers of hexachlorocyclohexane. aδ-1,2,3,4,5,6-Hexachlorocyclohexane is also an byproduct of insecticide Lindane (L465990) .
The Uses of LINDANE
Insecticide.
Definition
ChEBI: Beta-hexachlorocyclohexane is the beta-isomer of hexachlorocyclohexane. It has a role as a persistent organic pollutant. It is an organochlorine pesticide and a hexachlorocyclohexane.
General Description
Slightly musty odor.
Reactivity Profile
LINDANE may be incompatible with strong oxidizing and reducing agents. Incompatible with some amines, nitrides, azo/diazo compounds, with alkali metals, and with epoxides.
Health Hazard
ACUTE/CHRONIC HAZARDS: Highly toxic. May cause irritation on contact. Hazardous decomposition products.
Potential Exposure
The major commercial usage of BHC is based upon its insecticidal properties. α-BCH is used as an Agricultural chemical, pesticide, pharmaceutical, and veterinary drug. The 7-isomer has the highest acute toxic ity, but the other isomers are not without activity. It is gen erally advantageous to purify the 7-isomer from the less active isomers. The γ-isomer acts on the nervous system of insects, principally at the level of the nerve ganglia. As a result, lindane has been used against insects in a wide range of applications including treatment of animals, buildings, humans for ectoparasites, clothes; water for mosquitoes; living plants; seeds and soils. Some applications have been abandoned due to excessive residues, e.g., stored food stuffs. By voluntary action, the principal domestic producer of technical grade BHC requested cancellation of its BHC registrations on September 1, 1976. As of July 21, 1978, all registrants of pesticide products containing BHC voluntar ily canceled their registrations or switched their former BHC products to lindane formulations.
Environmental Fate
Biological. Dehydrochlorination of δ-BHC by a Pseudomonas sp. under aerobic con ditions was reported by Sahu et al. (1992). They also reported that when deionized water
containing δ-BHC was inoculated with Pseudomonas sp., the concentration of δ-BHC
decreased to undetectable levels after 8 days with concomitant formation of chloride ions
and δ-pentachlorocyclohexane. In four successive 7-day incubation periods, δ-BHC (5 and
10 mg/L) was recalcitrant to degradation in a settled domestic wastewater inoculum (Tabak
et al., 1981).
Chemical/Physical. δ-BHC dehydrochlorinates in the presence of alkalies. The hydrol ysis half-lives at pH values of 7 and 9 are 191 days and 11 hours, respectively (Worthing
and Hance, 1991).
Shipping
UN2761 Organochlorine pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explo sions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Decomposes on contact with powdered iron, aluminum, zinc, and on contact with strong bases producing trichlorobenzene.
Waste Disposal
A process has been developed for the destructive pyrolysis of benzene hexachloride @ 400 500℃ with a catalyst mixture which contains 5 10% of either cupric chloride, ferric chloride; zinc chloride; or aluminum chloride on activated carbon.
Properties of LINDANE
| Melting point: | 113-115 °C(lit.) |
| Boiling point: | 373.64°C (rough estimate) |
| Density | 1.7152 (rough estimate) |
| vapor pressure | 3.52 at 25 °C (Banerjee et al., 1990) |
| refractive index | 1.576-1.674 (589.3 nm 20℃) |
| Flash point: | 11 °C |
| storage temp. | 2-8°C |
| solubility | Soluble in ethanol, benzene, and chloroform (Weast, 1986) |
| form | Solid |
| BRN | 1907334 |
| Henry's Law Constant | (x 10-7 atm·m3/mol):
2.5 at 20 °C (approximate - calculated from water solubility and vapor pressure) |
| EPA Substance Registry System | .delta.-Hexachlorocyclohexane (319-86-8) |
Safety information for LINDANE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for LINDANE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran






You may like
-
 BENZENE HEXA CHLORIDE (BHC) 99%View Details
BENZENE HEXA CHLORIDE (BHC) 99%View Details -
 δ-BHC CAS 319-86-8View Details
δ-BHC CAS 319-86-8View Details
319-86-8 -
 δ-HCH CAS 319-86-8View Details
δ-HCH CAS 319-86-8View Details
319-86-8 -
 δ-HCH CAS 319-86-8View Details
δ-HCH CAS 319-86-8View Details
319-86-8 -
 δ-BHC CAS 319-86-8View Details
δ-BHC CAS 319-86-8View Details
319-86-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1