BHC
- CAS NO.:608-73-1
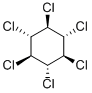
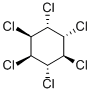
- Empirical Formula: C6H6Cl6
- Molecular Weight: 290.83
- MDL number: MFCD00003825
- EINECS: 210-168-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-15 10:43:25
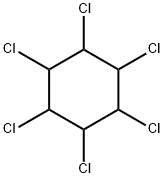
What is BHC?
Chemical properties
BHC is a white-to-brownish crystalline solid with a musty, phosgene-like odor.
The Uses of BHC
Insecticide.
Definition
An environmentally persistent constituent of a wide variety of agricultural, medical, and veterinary products.
General Description
White to yellow powder or flakes. Musty odor. The gamma isomer is known as lindane, a systemic insecticide. Toxic by ingestion or inhalation.
Reactivity Profile
Halogenated aliphatic compounds, such as HEXACHLOROCYCLOHEXANE, are moderately or very reactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Materials in this group may be incompatible with strong oxidizing and reducing agents. Also, they may be incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides.
Hazard
Highly toxic; questionable carcinogen.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, and tumorigenic data by ingestion and skin contact. Poison by ingestion, skin contact, and subcutaneous routes. Human systemic effects by inhalation: headache, nausea or vomiting, and fever. Implicated in aplastic anemia. Experimental reproductive effects. Mutation data reported. Lindane is more toxic than DDT or dieldrin. Potentially violent reaction with dlmethylformamide + iron. When heated to decomposition it emits highly toxic fumes of phosgene, HCl, and Cl-. See other benzenehexachloride entries. A toxic organochlorine that is persistent in the environment and accumulates in mammalian tissue. For cattle, the oral LD50 <= 100 mg/kg. The various isomers have dlfferent actions; the y (lindane) and a isomers are central nervous system stimulants, the principal symptom being convulsions. The p and A isomers are central nervous system depressants. The use of thermal vaporizers with lindane has caused acute poisoning by inhalation. mixture has been estimated at about 30 g and the dangerous dose of lindane at about 7 to 15 g. However, as already mentioned, a single dose of 45 mg (or approximately 0.65 mg/kg) of lindane caused convulsions. Lindane shows a marked dfference in toxicity to dfferent species. Its toxic effect on laboratory animals compares favorably with that of DDT, but for several domestic animals, notably calves, hdane is more toxic than DDT or Qeldrin. On a chronic systemic basis the a, p and y isomers are experimental carcinogens. Has been implicated in aplastic anemia. Dermatitis and perhaps other manifestations based on sensitivity represent a sort of chronic, though probably not systemic intoxication, which has been observed in humans. The signs and symptoms of confirmed acute poisoning in humans have paralleled those in experimental animals. These signs and symptoms are: excitation, hyperirritability, loss of equhbrium, clonictonic convulsions, and later depression.There is some evidence that the pulmonary edema and vascular collapse may be of neurogenic origm also. The symptoms in animals systemically poisoned by the yisomer alone are essentially similar to those caused by mixtures, although the onset may be earlier. Workers acutely exposed to high air concentrations of lindane and its decomposition products show headache, nausea, and irritation of eyes, nose, and throat. In rare instances, urticaria has followed exposure to lindane vapor. Unlike the signs and symptoms already mentioned, this allergic manifestation occurs only in susceptible individuals, and usually only after a period of sensitization.
Potential Exposure
The major commercial usage of BHC is based upon its insecticidal properties. α-BCH is used as an Agricultural chemical, pesticide, pharmaceutical, and veterinary drug. The 7-isomer has the highest acute toxic ity, but the other isomers are not without activity. It is gen erally advantageous to purify the 7-isomer from the less active isomers. The γ-isomer acts on the nervous system of insects, principally at the level of the nerve ganglia. As a result, lindane has been used against insects in a wide range of applications including treatment of animals, buildings, humans for ectoparasites, clothes; water for mosquitoes; living plants; seeds and soils. Some applications have been abandoned due to excessive residues, e.g., stored food stuffs. By voluntary action, the principal domestic producer of technical grade BHC requested cancellation of its BHC registrations on September 1, 1976. As of July 21, 1978, all registrants of pesticide products containing BHC voluntar ily canceled their registrations or switched their former BHC products to lindane formulations.
Carcinogenicity
A bioassay of lindane of the NCI
was published in 1977. The compound was administered
for 80 weeks to groups of Osborne–Mendel rats and
B6C3F1 mice. Time-weighted dietary levels for male rats
were 236 or 472 ppm; for female rats, 135 or 270 ppm; and for
male and female mice, 80 or 160 ppm. Results were as
follows: in rats, no tumors occurred at a statistically
significant incidence in the treated groups of either sex but
(2) in mice, the incidence of hepatocellular carcinoma in lowdose
males was significant when compared with that in the
pooled controls (controls 5/49, low dose 19/49, p = 0.001).
This finding, by itself, is insufficient to establish the carcinogenicity
of lindane. The incidence of hepatocellular carcinoma
in high-dose male mice was not significantly
different from that in matched or pooled controls. It is
concluded that under the conditions of this bioassay lindane
was not carcinogenic for Osborne–Mendel rats or B6C3F1
mice.
As with most other chlorinated insecticides, lindane has
been shown to exhibit tumor promotional activity, possibly
via inhibition of intercellular communication. Lindane
exposures do inhibit formation of liver tumors following
exposures to aflatoxin B1, suggesting an activity other than
tumor promotion for lindane. Enhancement of enzymatic
deactivation of aflatoxin via induction of microsomal
enzymes may play a role in this phenomenon.
The combination of lindane’s lack of significant mutagenic
activity, its tumor promotional properties, and the lack
of epidemiologic evidence suggest that lindane poses a
minimal to nonexistent risk of cancer to humans under
reasonable and prescribed use conditions.
Shipping
UN2761 Organochlorine pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explo sions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Decomposes on contact with powdered iron, aluminum, zinc, and on contact with strong bases producing trichlorobenzene.
Waste Disposal
A process has been developed for the destructive pyrolysis of benzene hexachloride @ 400 500℃ with a catalyst mixture which contains 5 10% of either cupric chloride, ferric chloride; zinc chloride; or aluminum chloride on activated carbon.
Properties of BHC
| Melting point: | 112.75°C |
| Boiling point: | 373.64°C (rough estimate) |
| Density | 1.8700 |
| refractive index | 1.5691 (estimate) |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Powder or Flakes |
| color | White,crystalline powder |
| Water Solubility | 31.4mg/L(25 ºC) |
| EPA Substance Registry System | 1,2,3,4,5,6-Hexachlorocyclohexane (608-73-1) |
Safety information for BHC
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H312:Acute toxicity,dermal H351:Carcinogenicity H362:Reproductive toxicity, effects on or via lactation H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P263:Avoid contact during pregnancy/while nursing. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for BHC
Abamectin manufacturer
A.B.ENTERPRISES
AB Enterprises
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
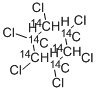
![HEXACHLOROCYCLOHEXANE, [14C(U)]](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB2200552.gif)

You may like
-
 608-73-1 1,2,3,4,5,6-Hexachlorocyclohexane 98%View Details
608-73-1 1,2,3,4,5,6-Hexachlorocyclohexane 98%View Details
608-73-1 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4