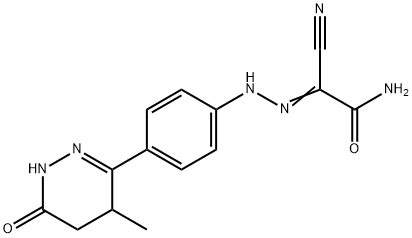
Levosimendan
Synonym(s):(-)-OR-1259;(R)-Simendan;2-[2-[4-[(4R)-1,4,5,6-Tetrahydro-4-methyl-6-oxo-3-pyridazinyl]phenyl]hydrazinylidene]-propanedinitrile;OR 1259;R)-((4-(1,4,5,6-Tetrahydro-4-methyl-6-oxo-3-pyridazinyl)phenyl)hydrazono) propanedintrile
- CAS NO.:141505-33-1
- Empirical Formula: C14H12N6O
- Molecular Weight: 280.28
- MDL number: MFCD00867135
- EINECS: 663-528-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-15 14:23:52
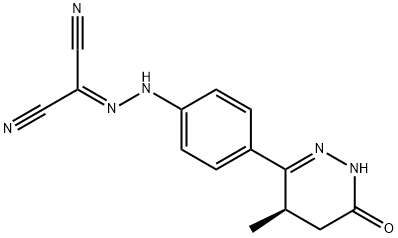
What is Levosimendan?
Absorption
The bioavailability of oral levosimendan is 85 ± 6% in healthy volunteers and 84 ± 4% in patients.
Description
Levosimendan was introduced in Sweden as an i.v. infusion for the treatment of acute heart failure or refractory symptoms of chronic heart failure in cases where conventional treatment (e.g., diuretic or ACE inhibitor) is not sufficient. Levosimendan is the (R)- enantiomer of simendan that belongs to the same class as pimobendan (Boehringer Ingelheim). Levosimendan is an innovative myofilament calcium sensitizer that increases myocardial contractility by selectively binding to the N-terminus of troponin C and by stabilizing the Ca2+-bound conformation of this contractile protein. It also activates ventricular and arterial adenostne triphosphate-regulated potassium channels which causes vasodilatation in vascular smooth muscle and protects myocardium against infarction. Its low phosphodiesterase III inhibiting activity is probably not responsible for its positive inotropic, lusitropic and dilating effects. Unlike other cardiotonic drugs, levosimendan is able to produce positive inotropic effects without prolonging myocardial relaxation or increasing the incidence of malignant arrythmias. It was clinically shown to have a lower risk of mortality in patients with heart failure when compared to placebo and dobutamine. Since it has a larger potential, levosimendan is currently under further clinical evaluation as a chronic treatment for congestive heart failure.
Chemical properties
Yellow Crystalline Powder
Originator
Orion (Finland)
The Uses of Levosimendan
Levosimendan has been used for screening its anti-human immunodeficiency virus type 1 (HIV-1) property. It has also been used for screening its cytotoxic effects in TP53 mutant and wild-type lung adenocarcinoma cell lines
The Uses of Levosimendan
Levosimendan is a positive inotropic agent that acts by sensitising troponin C to Ca2+, prolonging actin-myosin cross-bridge formation and therefore increasing contractility. This is an energy-independent process and therefore does not increase myocardial oxygen demand. Levosimendan also causes vasodilatation by opening ATP-sensitive K+ channels in vascular smooth muscle, reducing pre- and afterload and improving myocardial oxygen supply. It may have a role in the management of acute heart failure and postresuscitation myocardial dysfunction.
Indications
For short term treatment of acutely decompensated severe chronic heart failure (CHF). Also being investigated for use/treatment in heart disease.
Background
Levosimendan increases calcium sensitivity to myocytes by binding to troponin C in a calcium dependent manner. This increases contractility without raising calcium levels. It also relaxes vascular smooth muscle by opening adenosine triphosphate sensitive potassium channels. Levosimendan is used to manage acutely decompensated congestive heart failure.
What are the applications of Application
Levosimendan is a Ca2+ sensitizer that increases contractile force of the myocardium
Definition
ChEBI: Levosimendan is a hydrazone, a pyridazinone and a nitrile. It has a role as a vasodilator agent, an EC 3.1.4.17 (3',5'-cyclic-nucleotide phosphodiesterase) inhibitor, a cardiotonic drug and an anti-arrhythmia drug.
brand name
Simdax (Orion Pharmaceutica, Finland).
General Description
Levosimendan is a calcium sensitizer that can cause increased cardiac contractility by binding troponin C (EC50 = 9 nM), promotes vasodilation by activating ATP-sensitive potassium channels on vascular smooth muscle cells (EC50 = 0.28 μM), and performs a cardioprotective function by prompting the opening of mitochondrial potassium channels in cardiomyocytes. It also has been reported to inhibit phosphodiesterases 3 and 4 in left ventricular cardiac tissue with IC50 values of 2.5 nM and 25 μM, respectively.
Biochem/physiol Actions
Levosimendan has a potential to inhibit both acute human immunodeficiency virus type 1 (HIV-1) replication and the reactivation of latent HIV-1 proviruses. Therefore, it is considered to be a promising anti-HIV-1 agent.
Pharmacokinetics
Levosimendan is a new Ca2+-sensitizing inotropic agent. Ca2+ sensitizers represent a new class of inotropic agents, which overcome the disadvantages associated with currently available inotropic agents in as they are not associated with an increased risk of arrhythmias, cell injury and death due to Ca2+ overload in myocardial cells; they do not increase the activation energy; and they have the potential to reverse contractile dysfunction under pathophysiologic conditions, such as acidosis or myocardial stunning. Levosimendan has not been approved for use in the U.S. or Canada.
Clinical Use
Levosimendan is the bioactive enantiomer of racemate of Simendan. It acts as a positive inotropic agent with vasodilating activity. Levosimendan functions as a cardiotonic agent, promoting cardiac function and increasing blood vessel dilation. Levosimendan has been used for screening its anti-human immunodeficiency virus type 1 (HIV-1) property. It has also been used for screening its cytotoxic effects in TP53 mutant and wild-type lung adenocarcinoma cell lines.
Metabolism
Complete metabolism, with some active metabolites (OR-1855 and OR-1896) possibly extending the drug's haemodynamic effects.
Mode of action
Levosimendan is an innovative myofilament calcium sensitizer that increases myocardial contractility by selectively binding to the N-terminus of troponin C and by stabilizing the Ca2+-bound conformation of this contractile protein. It also activates ventricular and arterial adenostne triphosphate-regulated potassium channels which causes vasodilatation in vascular smooth muscle and protects myocardium against infarction. Its low phosphodiesterase III inhibiting activity is probably not responsible for its positive inotropic, lusitropic and dilating effects. Unlike other cardiotonic drugs, levosimendan is able to produce positive inotropic effects without prolonging myocardial relaxation or increasing the incidence of malignant arrythmias. It was clinically shown to have a lower risk of mortality in patients with heart failure when compared to placebo and dobutamine. Since it has a larger potential, levosimendan is currently under further clinical evaluation as a chronic treatment for congestive heart failure.
Properties of Levosimendan
| Melting point: | 216-219°C (dec.) |
| alpha | D25 -566° (tetrahydrofurane/methanol) |
| Density | 1.33±0.1 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | DMSO: ≥20mg/mL |
| pka | 6.3(at 25℃) |
| form | powder |
| color | yellow |
| optical activity | [α]/D -500 to -650°, c = 0.5 in THF |
Safety information for Levosimendan
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H312:Acute toxicity,dermal H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Levosimendan
Levosimendan manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


![2-[2-[4-(2-Azidotetrahydro-3-Methyl-5-oxo-2-furanyl)phenyl]hydrazinylidene]propanedinitrile (Mixture of DiasteroMers)](https://img.chemicalbook.in/CAS/GIF/252638-01-0.gif)
You may like
-
 141505-33-1 Levosimendan 98%View Details
141505-33-1 Levosimendan 98%View Details
141505-33-1 -
 141505-33-1 99%View Details
141505-33-1 99%View Details
141505-33-1 -
 Levosimendan 98%View Details
Levosimendan 98%View Details
141505-33-1 -
 Levosimendan CAS 141505-33-1View Details
Levosimendan CAS 141505-33-1View Details
141505-33-1 -
 Levosimendan CAS 141505-33-1View Details
Levosimendan CAS 141505-33-1View Details
141505-33-1 -
 Levosimendan CAS ID: 141505-33-1View Details
Levosimendan CAS ID: 141505-33-1View Details
141505-33-1 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
