LEVOCABASTINE
- CAS NO.:79516-68-0
- Empirical Formula: C26H29FN2O2
- Molecular Weight: 420.52
- MDL number: MFCD00672639
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 23:16:16
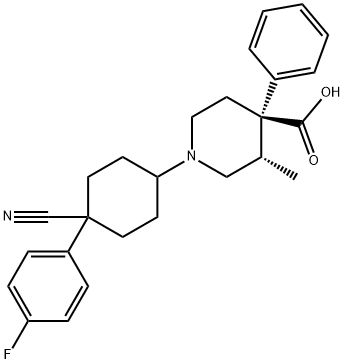
What is LEVOCABASTINE?
Absorption
After instillation in the eye, levocabastine is systemically absorbed, albeit at low levels.
Toxicity
Adverse effects include visual disturbances, dry mouth, cough, nausea, eyelid edema and lacrimation.
Originator
Livostin,Janssen-Cilag,Belgium
The Uses of LEVOCABASTINE
Anti histaminic.
Background
Levocabastine is a selective second-generation H1-receptor antagonist used for allergic conjunctivitis. Levocabastine was discovered at Janssen Pharmaceutica in 1979.
Indications
As an ophthalmic for the temporary relief of the signs and symptoms of seasonal allergic conjunctivitis. Also used as a nasal spray for allergic rhinitis.
Definition
ChEBI: Levocabastine is a member of piperidines.
Manufacturing Process
4-Cyano-4-(4-fluorophenyl)-heptanedioic acid diethyl ester is obtained by addition of ethyl acrylate to the anion from p-fluorophenylacetonitrile. By base catalyzed cyclization of these diester (sodium methoxide, 60°C, xylene) is synthesized an intermediate that after decarboethoxylation gives 1-(4- fluorophenyl)-4-oxycyclohexanecarbonitrile. By condensation of 3-methyl-5- phenylpiperidine-4-carboxylic acid benzyl ester and 1-(4-fluorophenyl)-4- oxycyclohexanecarbonitrile under reductive hydrogenation conditions (palladium-on-charcoal catalyst, 50°C, in ethanol) is prepared benzyl ester 4- piperidinecarboxylic acid, 1-(4-cyano-4-(4-fluorophenyl)cyclohexyl)-3-methyl- 4-phenyl-, (3S-(1(cis),3α,4β))-. The benzyl protecting group is then removed by hydrogenation method and 4-piperidinecarboxylic acid, 1-(4-cyano-4-(4- fluorophenyl)cyclohexyl)-3-methyl-4-phenyl-, (3S-(1(cis),3α,4β))- obtained is transformed into 4-piperidinecarboxylic acid, 1-(4-cyano-4-(4-fluorophenyl) cyclohexyl)-3-methyl-4-phenyl-, hydrochloride, (3S-(1(cis), 3α,4β))- (Levocabastine hydrochloride)
brand name
Livostin (Novartis).
Therapeutic Function
Antihistaminic
Pharmacokinetics
Levocabastine is a selective histamine H1-receptor antagonist exerting inhibitory effects on the release of chemical mediators from mast cells and on the chemotaxis of polymorphonuclear leukocytes and eosinophils. Both histamine and antigens induced conjunctivitis can be inhibited by levocabastine. Levocabastine can also reduce symptoms of allergic rhinitis by preventing an increase in vascular permeability of nasal mucosa.
Metabolism
Mostly unchanged. 10 to 20% is metabolized to the acylglucuronide of levocabastine.
Properties of LEVOCABASTINE
| Boiling point: | 589.9±50.0 °C(Predicted) |
| Density | 1.23 |
| pka | 3.57±0.40(Predicted) |
Safety information for LEVOCABASTINE
Computed Descriptors for LEVOCABASTINE
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1