LeterMovir
- CAS NO.:917389-32-3
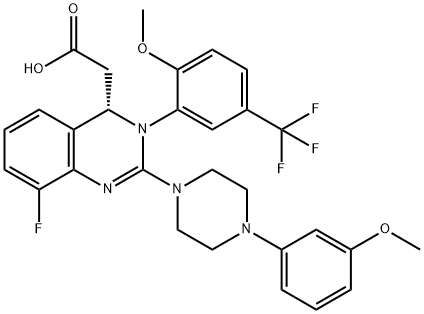
- Empirical Formula: C29H28F4N4O4
- Molecular Weight: 572.55
- MDL number: MFCD22572725
- EINECS: 200-001-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is LeterMovir?
Absorption
Letermovir has a bioavailability of 94% in healthy subjects when administered without cyclosporin, 35% in HSCT recipients when administered without cyclosporin, and 85% in HSCT recipients when administered with cyclosporin.
Letermovir's Tmax is 45 min to 2.25 hours. Time to steady state has been observed to be 9-10 days.
Taking Letermovir with food increases Cmax by an average of 129.82% (range of 104.35%-161.50%). No significant effect on AUC has been observed.
Toxicity
There is no human data on the safety of Letemovir in pregnancy. Embryo-fetal toxicity and malformations have been observed in rats at exposures 11 times the human exposure at the recommended human dose (RHD) of Letemovir. No such toxicity was noted in rats at 3 times human exposure at the RHD or in rabbits at values less than human exposure with the RHD. Total litter loss was observed in 21.7% of female rats at 2 times human exposure at RHD. This did not occur at values similar to human exposure at RHD.
No human data is available regarding lactation. Letemovir has been observed in the milk of lactating rats and in the blood of their nursing pups.
No data is available concerning the effect of Letemovir on human fertility. Testicular toxicity leading to reduced fertility has been observed in male rats.
No antidote exists for Letemovir overdosage. The effectiveness of dialysis in clearing plasma of Letemovir is unknown.
The Uses of LeterMovir
Letermovir acts as an orally or i.v administered cytomegalovirus terminus complex inhibitor, antiviral agent, used in the treatment of CMV infection which is prevalent in those with immunocompromised systems.
Indications
Letermovir is indicated for prophylaxis against cytomegalovirus (CMV) infection and disease in adult recipients of an allogeneic hematopoietic stem cell transplant (HSCT) who are CMV-seropositive. It is also indicated for prophylaxis against CMV disease in adult kidney transplant recipients who are at risk (i.e. donor CMV-seropositive/recipient CMV-seronegative).
Background
Letermovir recieved approval from the FDA on November 8th, 2017 for use in prophylaxis of cytomegalovirus (CMV) infection in allogeneic hematopoietic stem cell transplant patients. It is the first of a new class of CMV anti-infectives called DNA terminase complex inhibitors. Letermovir has recieved both priority and orphan drug status from the FDA. It is currently marketed under the brand name Prevymis.
Pharmacokinetics
Letermovir inhibits the activity of the DNA terminase complex of CMV thereby preventing the cutting of viral DNA into mature length genomes for packaging into viral particles. Letemovir inhibits the DNA terminase complex with an EC50 of 2.1nM.
Metabolism
Letermovir undergoes a minor degree of metabolism through UGT1A1/1A3.
Properties of LeterMovir
| Boiling point: | 706.5±70.0 °C(Predicted) |
| Density | 1.37±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | ≥57.3 mg/mL in DMSO; insoluble in EtOH; insoluble in H2O |
| form | solid |
| pka | 4.00±0.10(Predicted) |
| color | Off-white to yellow |
Safety information for LeterMovir
Computed Descriptors for LeterMovir
| InChIKey | FWYSMLBETOMXAG-QHCPKHFHSA-N |
| SMILES | N1C2=C(C=CC=C2F)[C@H](CC(O)=O)N(C2=CC(C(F)(F)F)=CC=C2OC)C=1N1CCN(C2=CC=CC(OC)=C2)CC1 |
Abamectin manufacturer
Zydus Lifesciences Ltd.
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

![2-Propenoic acid, 3-[3-fluoro-2-[[[4-(3-methoxyphenyl)-1-piperazinyl][[2-methoxy-5-(trifluoromethyl)phenyl]imino]methyl]amino]phenyl]-, methyl ester, (2E)-](https://img.chemicalbook.in/CAS/20180629/GIF/1791412-04-8.gif)
![2-Propenoic acid, 3-[3-fluoro-2-[[[[2-methoxy-5-(trifluoromethyl)phenyl]amino]carbonyl]amino]phenyl]-, methyl ester, (2E)-](https://img.chemicalbook.in/CAS/20180703/GIF/917389-26-5.gif)
You may like
-
 917389-32-3 Letermovir 98%View Details
917389-32-3 Letermovir 98%View Details
917389-32-3 -
 917389-32-3 98%View Details
917389-32-3 98%View Details
917389-32-3 -
 Letermovir CAS 917389-32-3View Details
Letermovir CAS 917389-32-3View Details
917389-32-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4