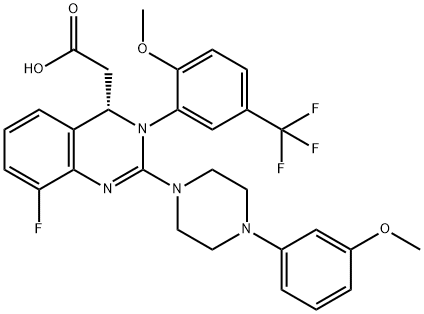
COMPUTED DESCRIPTORS
| Molecular Weight | 572.5 g/mol |
|---|---|
| XLogP3 | 4.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 7 |
| Exact Mass | 572.20466804 g/mol |
| Monoisotopic Mass | 572.20466804 g/mol |
| Topological Polar Surface Area | 77.8 Ų |
| Heavy Atom Count | 41 |
| Formal Charge | 0 |
| Complexity | 931 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Letermovir recieved approval from the FDA on November 8th, 2017 for use in prophylaxis of cytomegalovirus (CMV) infection in allogeneic hematopoietic stem cell transplant patients. It represents the first entry into a new class of CMV anti-infectives, DNA terminase complex inhibitors. Letermovir has recieved both priority and orphan drug status from the FDA. It is currently marketed under the brand name Prevymis.