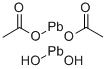
Lead acetate
- CAS NO.:301-04-2
- Empirical Formula: C4H6O4Pb
- Molecular Weight: 325.29
- MDL number: MFCD00012452
- EINECS: 206-104-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 17:10:30
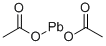
What is Lead acetate?
Description
Lead acetate is stable under ordinary conditions of use and storage. Lead acetate is incompatible with bromates, phenol, chloral hydrate, sulphides, hydrogen peroxide, resorcinol, salicylic acid, sulphites, vegetable infusions, alkalis, tannin, phosphates, citrates, chlorides, carbonates, tartrates, and acids. Lead (II) acetate, as well as white lead, has been used in cosmetics throughout history, though this practice has ceased in Western countries. It is still used in men’s hair colouring. Lead (II) acetate paper is used to detect the poisonous gas hydrogen sulphide. The gas reacts with lead (II) acetate on the moistened test paper to form a grey precipitate of lead (II) sulphide.
Description
Lead(II) acetate [Pb(OAc)2] is an inorganic salt with several uses, notably as an ingredient in dyes and mordants. Even though it is highly toxic (see the hazard information table), in the past it has been used as a sweetener and preservative in wines and other foods. It is commercially available as the anhydrous salt or the trihydrate1.
One of the earliest literature mentions of Pb(OAc)2 was in the 21st article in an 1878 series about polyiodides (e.g., KI3) by George Stillingfleet Johnson at King’s College London. Several articles from the 1890s cite Pb(OAc)2 in tests for whether it precipitates the anions of sodium or potassium salts under discussion. One of the salts was derived from what Pietro Biginelli at the University of Florence called “saligeninoxyacetic acid”, now more properly named 2-[2-(hydroxymethyl)phenoxy]acetic acid2.
Pb(OAc)2 can be made by boiling metallic lead in acetic acid in the presence of an oxidant; dissolving lead(II) oxide in acetic acid; or treating copper(II) acetate with the metal. The anhydrous salt and the trihydrate both have monoclinic crystal structures.
1. CAS Reg. No. 6080-56-4.
2. CAS Reg. No. 97388-49-3.
Chemical properties
Lead acetate is a white, flaky crystalline substance with a slight odor of acetic acid. Commercial grades may be powdered granules, or brown or gray lumps. Diacetate: Powder.
Chemical properties
Lead (II) acetate (Pb (CH3COO)2), also known as lead acetate, lead diacetate, plumbous acetate, sugar of lead, lead sugar, salt of Saturn, and Goulard's powder, is a white crystalline chemical compound with a sweetish taste. It is made by treating lead(II) oxide with acetic acid. Like other lead compounds, it is toxic. Lead acetate is soluble in water and glycerin. With water it forms the trihydrate, Pb(CH3COO)2·3H2O, a colorless or white efflorescent monoclinic crystalline substance.
The substance is used as a reagent to make other lead compounds and as a fixative for some dyes. In low concentrations, it is the principal active ingredient in progressive types of hair coloring dyes.[citation needed] Lead(II) acetate is also used as a mordant in textile printing and dyeing, as a drier in paints and varnishes, and in preparing other lead compounds.
The Uses of Lead acetate
2 – 1 - Sweetener
Like other lead (II) salts, lead (II) acetate has a sweet taste, which has led to its use as a sugar substitute throughout history. The ancient Romans, who had few sweeteners besides honey, would boil must (grape juice) in lead pots to produce a reduced sugar syrup called defrutum, concentrated again into sapa. This syrup was used to sweeten wine and to sweeten and preserve fruit. It is possible that lead(II) acetate or other lead compounds leaching into the syrup might have caused lead poisoning in anyone consuming it . Lead acetate is no longer used in the production of sweeteners in most of the world because of its recognized toxicity. Modern chemistry can easily detect it, which has all but stopped the illegal use that continued decades after legal use as a sweetener was banned .
2 – 1 - Sweetener2 – 1 – 1 - Resultant deaths
Pope Clement II died in October 1047. A toxicologic examination of his remains conducted in the mid – 20 th century confirmed centuries-old rumors that he had been poisoned with lead sugar.It is not clear if he was assassinated.
In 1787 painter Albert Christoph Dies swallowed, by accident, approximately 21 g of lead acetate. His recovery from this poison was slow and incomplete. He lived with illnesses until his death in 1822 .
Although the use of lead (II) acetate as a sweetener was already illegal at that time, composer Ludwig van Beethoven may have died of lead poisoning caused by wines adulterated with lead acetate.
Mary Seacole applied lead (II) acetate, among other remedies, against an epidemic of cholera in Panama.
The Uses of Lead acetate
Mordant in cotton dyes; lead coating for metals; drier in paints, varnishes and pigment inks; colorant in hair dyes. Weighting silks; manufacture of lead salts, chrome-yellow; as analytical reagent for detection of sulfide, determination of CrO3, MoO3.
Definition
ChEBI: A lead coordination entity in which a central lead(2+) atom is coordinated to two acetate ions.
Production Methods
Lead acetate is made by dissolving lead monoxide (litharge) or lead carbonate in strong acetic acid. Several types of basic salts are formed when lead acetates are prepared from lead monoxide in dilute acetic acid or at high pH. The basic salts of lead acetate are white crystalline compounds, which are highly soluble in water and dissolve in ethyl alcohol.
Lead acetate can be made by boiling elemental lead in acetic acid and hydrogen peroxide.
Flammability and Explosibility
Not classified
Potential Exposure
Lead acetate is used as a color additive in hair dyes; as a mordant in cotton dyes, in the lead coating of metals; as a drier in paints; varnishes and pigment inks; and in medicinals, such as astringents. Incompatibilities: A strong reducing agent. Reacts violently with strong oxidizers, bromates, strong acids; chemically active metals; phosphates, carbonates, phenols. Contact with strong acids forms acetic acid. Incompatible with strong bases: ammonia, amines, cresols, isocyanates, alkylene oxides; epichlorohydrin, sulfites, resorcinol, salicylic acid, and chloral hydrat
First aid
Skin Contact521: Flood all areas of body thathave contacted the substance with water. Do not wait toremove contaminated clothing; do it under the water stream.Use soap to help assure removal. Isolate contaminatedclothing when removed to prevent contact by others. EyeContact: Remove any contact lenses at once. Immediatelyflush eyes well with copious quantities of water or normalsalinefor at least 20- 30 min. Seek medicalattention.Inhalation: Leave contaminated area immediately; breathefresh air. Proper respiratory protection must be supplied toany rescuers. If coughing, difficult breathing, or any othersymptoms develop, seek medical attention at once, even ilsymptoms develop many hours affter exposure. Ingestion:Contact a physician, hospital, or poison center at once. Ifthe victim is unconscious or convulsing, do not inducevomiting or give anything by mouth. Assure that thepatient's airway is open and lay him on his side with hishead lower than his body and tran sport immediately to amedical facility. If conscious and not convulsing, give aglass of water to dilute the substance. V omiting should notbe induced without a physician's advice.Antidotes and special procedures for lead: Persons with sig-nificant lead poisoning are sometimes treated with CaEDTA while hospitalized. This“chelating” drug causes arush of lead from the body organs into the blood and kid-neys, and thus has its own hazards, and must be adminis-tered only by highly experienced medical personnel undercontrolled conditions and careful observation. Ca EDTA orsimilar drugs should never be used to prevent poisoningwhile exposure continues or without strict exposure control,as severe kidney damage can result.Note to physician: For severe poisoning BAL [British Anti-Lewisite, dimercaprol, dithiopropanol (C3HgOS2)] has beenused to treat toxic symptoms of certain heavy metals poi-soning. In the case of lead poisoning it may have SOMEvalue. Although BAL is reported to have a large margin ofsafety, caution must be exercised, because toxic effects maybe caused by excessive dosage. Most can be prevented bypremedication with 1-ephedrine sulfate (CAS: 134-72-5).
storage
Color Code- Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handlingandstorage. Store in a cool, dry place and keep tightly coveredand avoid contact with oxidizers, strong acids, chemicallyactive metals. A regulated, marked area should be estab-lished where this chemical is handled, used, or stored incompliance with OSHA Standard 1910.1045.
Shipping
UN1616 Lead acetate, Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Purification Methods
Crystallise it twice from anhydrous acetic acid and dry it under vacuum for 24hours at 100o. The solubility in H2O is 63% (at ~20o) and 200% (at boiling point). [Beilstein 2 IV 118.]
Incompatibilities
A strong reducing agent. Reacts violently with strong oxidizers, bromates, strong acids; chemically active metals; phosphates, carbonates, phenols. Contact with strong acids forms acetic acid. Incompatible with strong bases: ammonia, amines, cresols, isocyanates, alkylene oxides; epichlorohydrin, sulfites, resorcinol, salicylic acid, and chloral hydrate
Waste Disposal
Convert to nitrate using nitric acid; evaporate, then saturate with H2S; wash and dry the sulfide and ship to the supplier. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal
Precautions
Lead (II) acetate, as with any other lead salts, causes lead poisoning.
Properties of Lead acetate
| Melting point: | 75 °C (dec.)(lit.) |
| Boiling point: | decomposes at >280℃ [KIR78] |
| Density | 3.3 g/cm3 |
| vapor pressure | 15.7hPa at 25℃ |
| storage temp. | 2-8°C |
| solubility | 443 g/l (20 °c) |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Liquid |
| appearance | white crystals, granules, or powder |
| color | Clear colorless |
| Water Solubility | g/100g H2O: 19.7 (0°C), 55.2 (25°C); equilibrium solid phase, Pb(CH3COO)2 ·3H2O [KRU93]; g/100mL H2O: 44.3 (20°C), 221 (50°C) [KIR78] |
| λmax | 260nm(H2O)(lit.) |
| Merck | 14,5397 |
| Solubility Product Constant (Ksp) | pKsp: 2.75 |
| Dielectric constant | 2.5(0.0℃) |
| CAS DataBase Reference | 301-04-2(CAS DataBase Reference) |
| EPA Substance Registry System | Lead(II) acetate (301-04-2) |
Safety information for Lead acetate
Computed Descriptors for Lead acetate
Abamectin manufacturer
Kronox Lab Sciences Pvt Ltd
Nithyasri Chemicals (APURVA CHEMICALS)
L S Chemicals and Pharmaceuticals
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

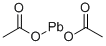
You may like
-
 301-04-2 LEAD ACETATE 99%View Details
301-04-2 LEAD ACETATE 99%View Details
301-04-2 -
 Lead acetate 99%View Details
Lead acetate 99%View Details -
 lead acetate CASView Details
lead acetate CASView Details -
 Lead acetate 99%View Details
Lead acetate 99%View Details -
 301-04-2 98%View Details
301-04-2 98%View Details
301-04-2 -
 Lead acetate 98%View Details
Lead acetate 98%View Details -
 Lead Acetate CASView Details
Lead Acetate CASView Details -
![Lead(II) Acetate [for Perovskite precursor] CAS 301-04-2](https://img.chemicalbook.in//Content/image/CP5.jpg) Lead(II) Acetate [for Perovskite precursor] CAS 301-04-2View Details
Lead(II) Acetate [for Perovskite precursor] CAS 301-04-2View Details
301-04-2