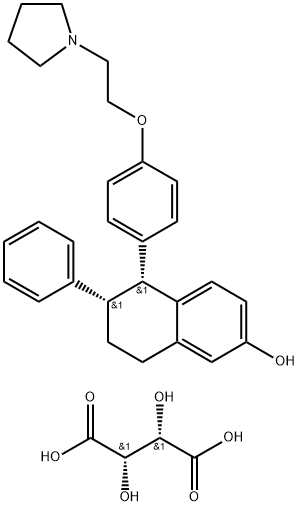
Lasofoxifene
- CAS NO.:180916-16-9
- Empirical Formula: C28H31NO2
- Molecular Weight: 413.55
- MDL number: MFCD09475650
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-08 09:03:09
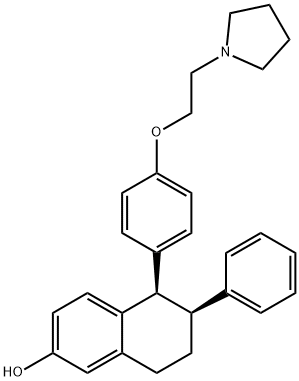
What is Lasofoxifene?
Absorption
Peak plasma concentrations (Cmax) were reached in about 6.0 to 7.3 hours . Displays higher oral bioavailability compared to other SERMs with increased resistance to intestinal glucuronidation due to nonpolar tetrahydronaphthalene structure . In a comparative study in the rat, lasofoxifene showed bioavailability of 62% .
Toxicity
Lasofoxifene increases the risk of venous thromboembolism driven by increased risk of deep vein thrombosis. Other adverse effects include hot flushes, muscle spasms and vaginal bleeding.
Chemical properties
Off-White Solid
The Uses of Lasofoxifene
A next-generation selective estrogen receptor modulator for treating disorders associated with increased bone turnover and osteopenia.
Background
Lasofoxifene is a non-steroidal 3rd generation selective estrogen receptor modulator (SERM) that selectively binds to both ERα and ERβ with high affinity. It is a naphthalene derivative marketed for prevention and treatment of osteoporosis and for the treatment of vaginal atrophy. It was initially developed as Oporia by Pfizer as a treatment for postmenopausal osteoporosis and vaginal atrophy, in which were both rejected for approval by FDA. Later Fablyn was developed as a result of a research collaboration between Pfizer and Ligand Pharmaceuticals with a newly submitted New Drug Application in 2008. It gained approval by European Commission in March 2009. Ligand Pharmaceuticals signed a license agreement with Sermonix Pharmaceuticals for the development and commercialization of oral lasofoxifene in the USA.
Indications
Investigated for use/treatment in postmenopausal osteoporosis to reduce the risk of both vertebral and novertebral fractures, as well as address other postmenopausal conditions, including reduction in risk of breast cancer and treatment of vulvar and vaginal atrophy (VVA)
What are the applications of Application
Lasofoxifene is a non-steroidal selective estrogen receptor modulator
Definition
ChEBI: Lasofoxifene is a member of the class of tetralins that is 5,6,7,8-tetrahydronaphthalen-2-ol in which the hydrogens at positions 5 and 6 are replaced by 4-[2-(pyrrolidin-1-yl)ethoxy]phenyl and phenyl groups, respectively (the 5R,6S-stereoisomer). It is a selective estrogen receptor modulator indicated for the prevention and treatment of osteoporosis in post-menopausal women. It has a role as an antineoplastic agent, a cardioprotective agent, an estrogen receptor agonist, an estrogen receptor antagonist and a bone density conservation agent. It is a member of tetralins, an aromatic ether, a member of naphthols and a N-alkylpyrrolidine.
Pharmacokinetics
Lasofoxifene exhibits both significant estrogenic and antiestrogenic activity both in vitro and in vivo, targeting any tissues that possess ERs, such as bone, uterus, breast, blood vessels, and liver. Binding assays demonstrated high affinity of the compound for both ERα and ERβ in a tissue-dependent manner. It mimics the effects of estradiol with varying agonist and antagonist effects.
Clinical Use
Lasofoxifene is a very potent second-generation SERM currently in phase III clinical trials for the treatment and prevention of osteoporosis in postmenopausal women.
Metabolism
Phase I oxidation via hepatic CYP3A4/CYP3A5 and CYP2D6 accounts for nearly half of total metabolism of lasofoxifene. Phase II conjugation reactions include glucuronidation and sulfation. Its glucuronidation is catalyzed by UGTs that are expressed in both the liver (UGT1A1, UGT1A3, UGT1A6, and UGT1A9) and the intestine (UGT1A8 and UGT1A10). Further metabolites of lasofoxifene detected in plasma are the glucuronide of a hydroxylated metabolite, and the methylated catechols .
Properties of Lasofoxifene
| Melting point: | 110-113°C |
| Boiling point: | 572.4±50.0 °C(Predicted) |
| Density | 1.150±0.06 g/cm3(Predicted) |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 10.31±0.60(Predicted) |
| color | White to Off-White |
Safety information for Lasofoxifene
Computed Descriptors for Lasofoxifene
New Products
4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION Methylcobalamin (vitamin B12) SODIUM METHYL PARABEN SODIUM VALPROATE AMOXICILLIN (AMOXYCILLIN) TRIHYDRATE ACICLOVIRRelated products of tetrahydrofuran



You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 1714107-48-8 5-Iodo-3-methyl-2-nitropyridine 98%View Details
1714107-48-8 5-Iodo-3-methyl-2-nitropyridine 98%View Details
1714107-48-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9