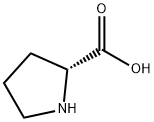
L-Hydroxyproline
Synonym(s):Hyp;Hydroxyproline;4-Hydroxyproline;L(-)-4-Hydroxypyrrolidine-2-carboxylic acid, Hyp;(2S,4R)-4-Hydroxypyrrolidine-2-carboxylic acid
- CAS NO.:51-35-4
- Empirical Formula: C5H9NO3
- Molecular Weight: 131.13
- MDL number: MFCD00064320
- EINECS: 200-091-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
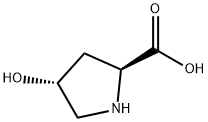
What is L-Hydroxyproline?
Description
A non-essential amino acid. Can be isolated from gelatin. Hydroxyproline has not been reported as added to food in any of the NAS surveys.
Chemical properties
White crystalline powder
The Uses of L-Hydroxyproline
A natural constituent of animal structural proteins such as collagen and elastin. Several microorganisms producing proline trans-4- and cis-3-hydroxylase were discovered and these enzymes were applied to the industrial production of trans-4- and cis-3-hydroxy-L-proline.
The Uses of L-Hydroxyproline
A versatile reagent for the synthesis of neuroexcitatory kainoids and antifungal echinocandins.
The Uses of L-Hydroxyproline
hydroxyproline is a skin-conditioning amino acid. It is a component of collagen.
What are the applications of Application
trans-4-Hydroxy-L-proline is a versatile reagent for the synthesis of neuroexcitatory kainoids and antifungal echinocandins
Definition
ChEBI: An optically active form of 4-hydroxyproline having L-trans-configuration.
Production Methods
In the past L-Hydroxyproline was isolated by hydrolysis of animal collagen, e. g. gelatine or collagen, of which it is a major constituent. L-Hydroxyproline is an unnatural amino acid which is made in the body by hydroxylation of L-Proline. The presence of L-Hydroxyproline and proline are key to maintaining the stability of the tight collagen helix.
Today L-Hydroxyproline is manufactured by bio-catalysed hydroxylation of proline in bacteria. Together with its other isomers, L-Hydroxyproline is also used as an inter mediate for a range of pharmaceutical active ingredients.
Produced by hydroxylation of L-proline after protein synthesis. It is contained rich in collagen and elastin. Known to stabilization of triple-helix structure of collagen. Widely used as an intermediate for medicines.
General Description
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
Biochem/physiol Actions
Trans-4-Hydroxy-L-proline is used in the organic synthesis of depsilairdin analogues, noncompetitive peptidomimetic inhibitors of multidrug resistance P-glycoprotein and 3-guaninyl-5-hydroxymethyl-2-pyrrolidinone (4) or 3-adeninyl-5-hydroxymethyl-2-pyrrolidinone (5) nucleoside analogues.
Purification Methods
Crystallise it from MeOH/EtOH (1:1). Separation from normal allo-isomer can be achieved by crystallisation of the copper salts [see Levine Biochemical Preparations 8 114 1961]. Separation from proline is achieved via the crystalline picrate, CdCl2, or acid ammonium rhodanate salts [see Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 p 2182 1961, Kapfhammer & Mohn Z Physiol Chem 306 76 1956]. [Beilstein 22/5 V 7.]
Properties of L-Hydroxyproline
| Melting point: | 273 °C (dec.)(lit.) |
| Boiling point: | 242.42°C (rough estimate) |
| alpha | -75.5 º (c=5, H2O) |
| Density | 1.3121 (rough estimate) |
| vapor density | 4.5 (vs air) |
| refractive index | -75.5 ° (C=4, H2O) |
| storage temp. | Store below +30°C. |
| solubility | H2O: 50 mg/mL |
| form | Crystals or Crystalline Powder |
| pka | 1.82, 9.66(at 25℃) |
| color | White |
| Odor | Odorless |
| PH | 5.5-6.5 (50g/l, H2O, 20℃) |
| optical activity | [α]25/D 75.6°, c = 1 in H2O |
| Water Solubility | 357.8 g/L (20 º C) |
| Merck | 14,4840 |
| BRN | 471933 |
| CAS DataBase Reference | 51-35-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Hydroxyproline(51-35-4) |
| EPA Substance Registry System | trans-4-Hydroxy-L-proline (51-35-4) |
Safety information for L-Hydroxyproline
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for L-Hydroxyproline
| InChIKey | PMMYEEVYMWASQN-DMTCNVIQSA-N |
L-Hydroxyproline manufacturer
BTC pharm India
ARRAKIS INDUSTRIES LLP
Archerchem Healthcare Pvt., Ltd. (part of Archerchem Group)
Chemfield Inc
ASM Organics
New Products
Trans-methyl 4-aminocyclohexane- carboxylate HCl 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-Iodophenylacetic acid (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine Cyclohexane, (2-propynyloxy)- 3-Bromobenzaldehyde, 95% 2-Naphthol, 98% Cysteamine hydrochloride, 98% Copper(II) bromide, 98% 1-Chloro-2,4-difluorobenzene,98% Dodecylbenzenesulfonic acid, 95% L-Glycine methyl ester.HCl Fmoc-L-Tyr(tBu)-OH Calcium Alphaketoglutarate* H-Ser(t-Bu)-Ser(t-Bu)-Gly-OH Fmoc-Ser(tBu)-Ser(Ψ(Me,Me)pro-OH Triphosgene 5-Cyanophthalide 10-Methoxy-5H-dibenz[b,f]azepine L-Glutamic Acid Dimethyl Ester Hcl 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base)Related products of tetrahydrofuran



You may like
-
 L-Hydroxyproline 51-35-4 98+View Details
L-Hydroxyproline 51-35-4 98+View Details
51-35-4 -
 trans-4-Hydroxy-L-proline CAS 51-35-4View Details
trans-4-Hydroxy-L-proline CAS 51-35-4View Details
51-35-4 -
 L-Hydroxy proline CAS 51-35-4View Details
L-Hydroxy proline CAS 51-35-4View Details
51-35-4 -
 trans-4-Hydroxy-L-proline CAS 51-35-4View Details
trans-4-Hydroxy-L-proline CAS 51-35-4View Details
51-35-4 -
 L-Hydroxyproline CASView Details
L-Hydroxyproline CASView Details -
 L-HYDROXYPROLINE For Synthesis CAS 51-35-4View Details
L-HYDROXYPROLINE For Synthesis CAS 51-35-4View Details
51-35-4 -
 trans-4-Hydroxy-L-proline 98% CAS 51-35-4View Details
trans-4-Hydroxy-L-proline 98% CAS 51-35-4View Details
51-35-4 -
 Hydroxyproline CAS 51-35-4View Details
Hydroxyproline CAS 51-35-4View Details
51-35-4
