L-Homocystine
- CAS NO.:626-72-2
- Empirical Formula: C8H16N2O4S2
- Molecular Weight: 268.35
- MDL number: MFCD00020391
- EINECS: 210-962-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
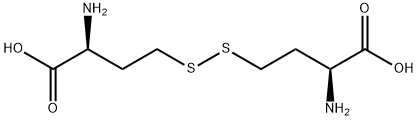
What is L-Homocystine?
Chemical properties
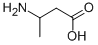
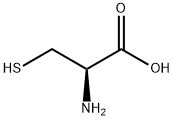
L-homocysteine is an aliphatic amino acid with the molecular formula (C8H16N2O4S2). It is a white powder with an acidic taste and is easily soluble in water. It has limited solubility in ethanol. L-homocysteine has a sulfhydryl group on its side chain, which allows it to form disulfide bonds with other L-homocysteine residues in proteins. This feature contributes to the stable three-dimensional structure of proteins. As a result, L-homocysteine finds extensive applications in industries such as pharmaceuticals, food, feed, and cosmetics.
The Uses of L-Homocystine
Increased levels lead to hyperhomocysteinemia; a cardiovascular risk factor in prediction of coronary heart disease as well as being associated with congenital birth defects, pregnancy complications and cancer.
Definition
ChEBI: L,L-homocystine is a homocystine in which both chiral centres have L configuration. It is functionally related to a L-homocysteine. It is a tautomer of a L,L-homocystine zwitterion.
Synthesis
L-homoserine(626-72-2) is biosynthesized from L-aspartate, which is synthesized from oxaloacetate, a TCA cycle intermediate. Homoserine is activated through esterification to form the O-acetyl ester by the enzyme homoserine O-acetyltransferase. In certain organisms, including the yeast Saccharomyces cerevisiae and certain bacteria, hydrogen sulfide reacts with O-acetyl-L-homoserine, replacing the acetyl group and forming L-homocysteine.
Purification Methods
The acid (3g) is dissolved in freshly boiled H2O (30mL) under N2, cooled under N2 (all operations should be under N2), absolute EtOH (100mL) is added, the acid is filtered off, and a second crop is obtained by diluting the filtrate to 500mL with absolute EtOH, kept overnight in a refrigerator, filtered, washed with EtOH and dried in a vacuum. The D(R,R)-form has similar properties but is –ve in M HCl and +ve in H2O. [du Vigneaud & Patterson J Biol Chem 109 101 1935, du Vigneaud & Brown Biochemical Preparations 5 93, 95 1975, Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 pp 2667-2670 1961, Beilstein 4 III 1643, 4 IV 3199, Koegel et al. J Am Chem Soc 77 5708 1955.]
Properties of L-Homocystine
| Melting point: | 281-284 °C (dec.)(lit.) |
| alpha | 77 º (c=1% in 1N HCl) |
| Boiling point: | 507.6±50.0 °C(Predicted) |
| Density | 1.443±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | H2O : 5 mg/mL (18.63 mM; ultrasonic and adjust pH to 3 with H2O)DMSO : 1 mg/mL (3.73 mM; ultrasonic and adjust pH to 5 with HCl) |
| form | Powder |
| pka | 1.90±0.10(Predicted) |
| color | Off-white to light yellow |
| BRN | 1728583 |
| CAS DataBase Reference | 626-72-2(CAS DataBase Reference) |
| EPA Substance Registry System | L-Homocystine (626-72-2) |
Safety information for L-Homocystine
Computed Descriptors for L-Homocystine
| InChIKey | ZTVZLYBCZNMWCF-WDSKDSINSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 L-Homocystine 95% CAS 626-72-2View Details
L-Homocystine 95% CAS 626-72-2View Details
626-72-2 -
 L-Homocystine CAS 626-72-2View Details
L-Homocystine CAS 626-72-2View Details
626-72-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1