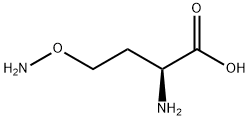
L-CANALINE BASE
- CAS NO.:496-93-5
- Empirical Formula: C4H10N2O3
- Molecular Weight: 134.13
- MDL number: MFCD00057642
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 16:48:35
What is L-CANALINE BASE?
Description
L-Canaline is an aminooxy analog of ornithine that irreversibly inhibits aminotransferases (transaminases), including ornithine aminotransferase (Ki = 2 μM). It forms oximes with α-keto acids and aldehydes, most notably with pyridoxal phosphate, an essential cofactor of aminotransferases. L-Canaline is naturally found in plants, including legumes, and is involved in the metabolism of L-canavanine, an aminooxy analog of arginine. It is cytotoxic to a range of organisms, including bacteria, insects, and parasites.
Definition
ChEBI: L-canaline is a non-proteinogenic L-alpha-amino acid that is L-homoserine in which the hydroxy group at position 4 is substituted by an aminooxy group. It has been isolated from legumes and plays an essential role in lugume chemical defense against insects. It has a role as a plant metabolite, an antineoplastic agent, an antimetabolite and a phytogenic insecticide. It is functionally related to a L-homoserine. It is a tautomer of a L-canaline zwitterion.
Synthesis Reference(s)
Tetrahedron, 23, p. 4441, 1967 DOI: 10.1016/S0040-4020(01)88842-6
in vitro
canaline strongly inhibited the activity of pyridoxal-dependent enzymes, including amino acid decarboxylases, 5-hydroxytryptophan decarboxylase, aminotransferases, tyrosine aminotransferase, ornithine transcarbamylase and plasma diamino-oxidase. the canaline inhibition was due to complex formation between canaline and the pyridoxal coenzyme. l-canaline is one of the most potent inhibitors of pyridoxal enzymes. the ic50 value of l-canaline against ornithine aminotransferase was 3 ×10-6m [4].
in vivo
intraperitoneal administration of 500 mg of dl-canaline/kg body wt. only produced a transient inhibition of oat in brain and liver by 65-70%, suggesting that dl-canaline was not a useful tool in studies of biological consequences of oat inhibition. [1].
References
[1] bolkenius f n, kndgen b, seiler n. dl-canaline and 5-fluoromethylornithine. comparison of two inactivators of ornithine aminotransferase[j]. biochemical journal, 1990, 268(2): 409-414.
[2] rosenthal g a, rhodes d. l-canavanine transport and utilization in developing jack bean, canavalia ensiformis (l.) dc.[leguminosae][j]. plant physiology, 1984, 76(2): 541-544.
[3] peraino c, bunville l g, tahmisian t n. chemical, physical, and morphological properties of ornithine aminotransferase from rat liver[j]. journal of biological chemistry, 1969, 244(9): 2241-2249.
[4] rahiala e l, kekomki m, jnne j, et al. inhibition of pyridoxal enzymes by l-canaline[j]. biochimica et biophysica acta (bba)-enzymology, 1971, 227(2): 337-343.
Properties of L-CANALINE BASE
| Density | 1.298 |
| storage temp. | 2-8°C |
| solubility | ≤1mg/ml in DMSO |
| form | crystalline solid |
| pka | pK1: 2.40;pK2: 3.70;pK3: 9.20 (25°C) |
| color | White to off-white |
Safety information for L-CANALINE BASE
Computed Descriptors for L-CANALINE BASE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4