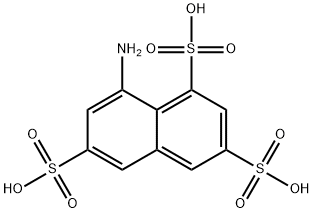
Koch acid
- CAS NO.:117-42-0
- Empirical Formula: C10H9NO9S3
- Molecular Weight: 383.37
- MDL number: MFCD00035857
- EINECS: 204-188-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-03 09:06:10
What is Koch acid?
Chemical properties
1-Aminonaphthalene-3,6,8-trisulfonic acid [117-42-0]. (8-aminonaphthalene-1,3,6- trisulfonic acid), Koch acid, T acid, C10H9NO9S3, Mr 383.36, and its alkali-metal salts are readily soluble in water. The solutions are unusual for this class of compound in that they exhibit no fluorescence. Hydrolysis with dilute sulfuric acid produces 1-hydroxynaphthalene-3,6,8-trisulfonic acid, whereas diazotization followed by acid hydrolysis results in formation of the corresponding sultone. Reduction with zinc and alkali desulfonates 1-Aminonaphthalene-3,6,8-trisulfonic acid to give 1-amino-naphthalene-3,6-disulfonic acid. Amination gives 1,3-diaminonaphthalene-6,8- disulfonic acid.
The Uses of Koch acid
Apart from caustic fusion to give H acid, the other main use is the production of chromotropic acid by treatment with strong caustic under pressure.
Production Methods
Naphthalene in sulfuric acid solution is treated with gradual addition of 65 % oleum and temperature profiling (to 160℃) to optimize formation of the 1,3,6-trisulfonic acid. A mixture of sulfuric acid and nitric acids is added slowly at 40℃ to form 1-nitronaphthalene-3,6,8-trisulfonic acid (nitro-Koch acid) which is converted to neutral liquor by liming out. The liquor is reduced with iron powder in the presence of acetic acid and, after removal of iron residues, the crude product is isolated, as the acid calcium – sodium salt. This is purified by dissolution with sodium carbonate and removal of calcium carbonate. The trisodium salt liquor is concentrated before direct use in H acid manufacture. To obtain pure Koch acid, the 1-nitro3,6,8-trisulfonic acid can be isolated before reduction. The reduction step can alternatively be carried out by hydrogenation of the aqueous solution of nitro-Koch acid in the presence of a nickel catalyst.
Properties of Koch acid
| Density | 1.974 |
| pka | -1.62±0.40(Predicted) |
| EPA Substance Registry System | 1,3,6-Naphthalenetrisulfonic acid, 8-amino- (117-42-0) |
Safety information for Koch acid
Computed Descriptors for Koch acid
Koch acid manufacturer
Viana Chemicals Private Limited
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

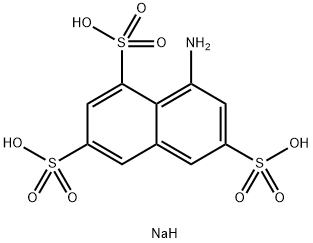
You may like
-
 117-42-0 KOCH ACID 99%View Details
117-42-0 KOCH ACID 99%View Details
117-42-0 -
 117-42-0 98%View Details
117-42-0 98%View Details
117-42-0 -
 8-Amino-1,3,6-naphthalenetrisulfonic acid 98%View Details
8-Amino-1,3,6-naphthalenetrisulfonic acid 98%View Details
117-42-0 -
 8-amino-1,3,6-naphthalenetrisulfonic acid(KOCH Acid)View Details
8-amino-1,3,6-naphthalenetrisulfonic acid(KOCH Acid)View Details
117-42-0 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
