1-Aminonaphthalene-6-sulfonic acid
- CAS NO.:119-79-9
- Empirical Formula: C10H9NO3S
- Molecular Weight: 223.25
- MDL number: MFCD00004030
- EINECS: 204-351-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
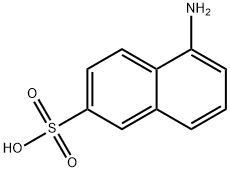
What is 1-Aminonaphthalene-6-sulfonic acid?
Chemical properties
Purple Solid. 1-Aminonaphthalene-6-sulfonic acid [119- 79-9]. (5-aminonaphthalene-2-sulfonic acid), 1,6-Cleve’s acid, C10H9NO3S, Mr 223.24, crystallizes from boiling water as plates of the dihydrate. Its sodium, potassium, and calcium salts are all readily soluble in water, but the barium salt is only sparingly soluble. Sulfonation with 10 % oleum yields 1-aminonaphthalene-4,6-disulfonic acid. Caustic fusion gives 5- amino-2-hydroxynaphthalene, and Bucherertype hydrolysis gives 1-hydroxynaphthalene-6- sulfonic acid.
The Uses of 1-Aminonaphthalene-6-sulfonic acid
Intermediate of dyestuff
The Uses of 1-Aminonaphthalene-6-sulfonic acid
Fluorescent reagent.
The Uses of 1-Aminonaphthalene-6-sulfonic acid
5-Amino-2-naphthalenesulfonic acid (1,6-Cleve′s acid) was used in the synthesis of nitrate reductase and detection of nitrate reduction by anaerobic bacteria.
Definition
ChEBI: 5-aminonaphthalene-2-sulfonic acid is an aminonaphthalenesulfonic acid.
Production Methods
Sulfonation of 1-naphthylamine with concentrated sulfuric acid at 130℃ gives mainly 1-aminonaphthalene-6-sulfonic acid. However, the latter is always made, together with the 1,7-isomer, by nitration of naphthalene-2-sulfonic acid to give the mixed 5- and 8- nitronaphthalene-2-sulfonic acids follwed by reduction to ‘‘mixed Cleve’s acids.’’ A b-sulfonation mass is diluted with sulfuric acid, then 67.5 % nitric acid is added slowly at 34℃. After being quenched and neutralized with calcium carbonate, the filtered liquor is reduced by boiling with iron powder, slight acidity being maintained with sulfuric acid. After basification with magnesia the iron residues are filtered off and the filtrate is evaporated until the magnesium salt of the product crystallizes on cooling. Filtration followed by washing with magnesium sulfate gives a 34 % yield of the magnesium salt of 1,6-Cleve’s acid. Purification by dissolution in hot water, acidification, and filtration of the free acid while hot reduce the yield to 25 % based on naphthalene.
General Description
The crystal structure of 5-amino-2-naphthalenesulfonic acid (1,6-Cleve′s acid) depicted the presence of a sulfonate-aminium group zwitterion.
Properties of 1-Aminonaphthalene-6-sulfonic acid
| Melting point: | >300°C |
| Density | 1.3588 (rough estimate) |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 1.6500 (estimate) |
| storage temp. | Refrigerator |
| solubility | soluble100mg/mL, clear, brown (1N NH4OH) |
| form | Solid |
| pka | pK1: 3.80 (25°C) |
| color | Purple |
| Water Solubility | 1g/L(16 ºC) |
| Merck | 14,2350 |
| BRN | 1819887 |
| CAS DataBase Reference | 119-79-9(CAS DataBase Reference) |
| NIST Chemistry Reference | 1-Naphthylamine-6-sulfonic acid(119-79-9) |
| EPA Substance Registry System | 2-Naphthalenesulfonic acid, 5-amino- (119-79-9) |
Safety information for 1-Aminonaphthalene-6-sulfonic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for 1-Aminonaphthalene-6-sulfonic acid
1-Aminonaphthalene-6-sulfonic acid manufacturer
Colour Finders
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 119-79-9 5-Amino-2-naphthalenesulfonic acid 98%View Details
119-79-9 5-Amino-2-naphthalenesulfonic acid 98%View Details
119-79-9 -
 5-Amino-2-naphthalenesulfonic Acid CAS 119-79-9View Details
5-Amino-2-naphthalenesulfonic Acid CAS 119-79-9View Details
119-79-9 -
 5-Aminonaphthalene-2-sulfonic acid CAS 119-79-9View Details
5-Aminonaphthalene-2-sulfonic acid CAS 119-79-9View Details
119-79-9 -
 5-Amino-2-naphthalenesulfonic acid CAS 119-79-9View Details
5-Amino-2-naphthalenesulfonic acid CAS 119-79-9View Details
119-79-9 -
 5-AMINO-2-NAPHTHALENE SULFONIC ACIDView Details
5-AMINO-2-NAPHTHALENE SULFONIC ACIDView Details
119-79-9 -
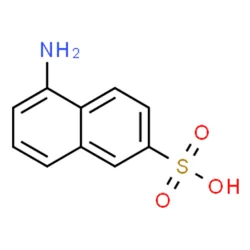 1,6-cleve's acidView Details
1,6-cleve's acidView Details
119-79-9 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
