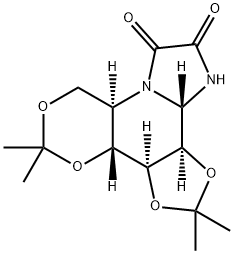
KIFUNENSINE
Synonym(s):FR 900494;Hexahydro-6R,7S,8aS-trihydroxy-5R-(hydroxymethyl)-imidazo[1,2-a] pyridine-2,3-dione;Kifunensine, Kitasatosporia kifunense - CAS 109944-15-2 - Calbiochem
- CAS NO.:109944-15-2
- Empirical Formula: C8H12N2O6
- Molecular Weight: 232.19
- MDL number: MFCD00270017
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-06 15:14:14
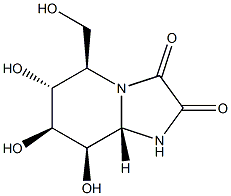
What is KIFUNENSINE?
Description
Historically isolated from Kitasatosporia kifunense.1?Kifunensine (CAS 109944-15-2) is an inhibitor of class I α-mannosidases which inhibit glycoprotein processing. Inhibits human endoplasmic reticulum α-1,2-mannosidase I and Golgi Class I mannosidases IA, IB and IC with Ki values of 130 and 23 nM respectively.2 Inhibition of endoplasmic reticulum a-mannosidase I activity rescues the human a-sarcoglycan R77C mutation suggesting a new pharmacological approach for limb girdle muscular dystrophy type 2D patients carrying mutations that impair a-sarcoglycan trafficking.3 Improves maturation of misfolded proteins.4
Chemical properties
White Powder
The Uses of KIFUNENSINE
Kifunensine is an alkaloid produced by the fungus, Kitasatosporia kifunense, and has been shown to be a weak inhibitor of aryl mannosidase. It is a unique oxamide derivative of mannojirimycin and a potent inhibitor of the glycoprotein processing enzyme
The Uses of KIFUNENSINE
Kifunensine is an alkaloid produced by the fungus, Kitasatosporia kifunense, and has been shown to be a weak inhibitor of aryl mannosidase. It is a unique oxamide derivative of mannojirimycin and a potent inhibitor of the glycoprotein processing enzyme mannosidase I. Kifunenesine is ineffective in inhibiting either mannosidase II or the endoplasmic reticulum (ER) a-mannosidase. It also possesses immunomodulating properties based on chemical, physicochemical and x-ray crystallographic analysis.When tested in cell culture using influenza virus-infected MDCK cells, kifunensine, at 1 mg/ml or less, caused almost complete inhibition of complex chain formation with the accumulation of Man9(GlcNAc)2. Thus, kifunensine was proven to be 50 to 100 times more effective than deoxymannojirimycin.Ph
What are the applications of Application
Kifunensine is an immunoactive cell adhesion inhibitor that displays competitive inhibition against immunosuppressive factor in mice
General Description
A potent alkaloid inhibitor of mannosidase I. Does not affect mannosidase II and the endoplasmic reticulum α-mannosidase.
Biological Activity
Inhibitor of class I α -mannosidases that inhibits glycoprotein processing. Inhibits human endoplasmic reticulum α -1,2-mannosidase I and Golgi Class I mannosidases IA, IB and IC with K i values of 130 and 23 nM respectively.
Biochem/physiol Actions
Product does not compete with ATP.
Storage
Store at -20°C
References
1)?Iwami et al. (1987), A new immunomodulator, FR-900494: taxonomy, fermentation, isolation, and physico-chemical and biological characteristics; J.Antibiot. (Tokyo) 40 612 2)?Elbein et al. (1990), Kifunensine, a potent inhibitor of the glycoprotein processing mannosidase I; ?J.Biol.Chem. 265 15599 3)?Bartoli et al. (2008), Mannosidase I inhibition rescues the human alpha-sarcoglycan R77C recurrent mutation; ?Hum.Mol.Genet. 17 1214 4)?Wang et al. (2011), Inhibition of endoplasmic reticulum-associated degradation rescues native folding in loss of function protein misfolding diseases; J.Biol.Chem. 286 43454
Properties of KIFUNENSINE
| Melting point: | >280°C |
| Density | 1.85±0.1 g/cm3(Predicted) |
| storage temp. | −20°C |
| solubility | water, double-distilled: soluble50MM |
| form | White solid |
| pka | 9.61±0.70(Predicted) |
| color | White |
| Water Solubility | Soluble to 5 mM in water with sonication |
| Stability: | Desiccate and Store at -20°C |
Safety information for KIFUNENSINE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for KIFUNENSINE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Kifunensine, Kitasatosporia kifunense CAS 109944-15-2View Details
Kifunensine, Kitasatosporia kifunense CAS 109944-15-2View Details
109944-15-2 -
 Kifunensine CAS 109944-15-2View Details
Kifunensine CAS 109944-15-2View Details
109944-15-2 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
