VERATRIDINE
Synonym(s):3-Veratroylveracevine;Veracevine veratrate;Veratridine - CAS 71-62-5 - Calbiochem
- CAS NO.:71-62-5
- Empirical Formula: C36H51NO11
- Molecular Weight: 673.79
- MDL number: MFCD16661233
- EINECS: 200-758-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-05 14:33:18
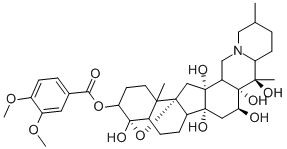
What is VERATRIDINE?
Description
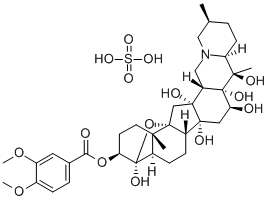
This steroidal alkaloid from Veratrum viride was given this name by Bosetti. It is almost certainly identical with the amorphous veratrine isolated by Schmidt and Koppen, Merck, and Wright and Luff. The base is a colourless, amorphous powder which melts over a wide range. It has [α]22D + 8.0° (EtOH) and yields a sulphate nonahydrate which forms colourless silky needles that resinify in air. The nitrate is only sparingly soluble in H2O. The solution of the alkaloid in H2SO4 becomes orange-red and eventually crimson with a blue fluorescence while that in HCI is colourless. Alkaline hydrolysis furnishes veratric acid and cevine.
The Uses of VERATRIDINE
Veratridine causes Na+ channels to stay open during a sustained membrane depolarization by abolishing inactivation.?This, in turn, opens voltage-activated calcium channels, thus increasing intracellular calcium content and inducing neurotransmitter release. Alkaloid neurotoxin which depolarizes excitable tissue; used to increase membrane sodium permeability.
What are the applications of Application
Veratridine is a voltage dependent Na+ channel opening agent
Definition
ChEBI: Veratridine is a steroid. It has a role as a sodium channel modulator. It is functionally related to a cevane.
General Description
Veratridine is one of several alkaloids isolated from the seeds of Schoenocaulon officinale and from the rhizome of Veratrum album. The crude extract is called veratrine or sabadilla and contains cevadine, veratridine, cevadilline, sabadine and cevine. It is a neurotoxin, which belongs to the family Liliaceae and genus, Schoenocaulon. Veratridine is soluble in lipid.
Biochem/physiol Actions
Opens voltage-dependent Na+ channels and prevents their inactivation. This, in turn, opens voltage-activated calcium channels, thus increasing intracellular calcium content and inducing neurotransmitter release. Alkaloid neurotoxin which depolarizes excitable tissue; used to increase membrane sodium permeability. Veratridine is cytotoxic to chromaffin cells in vitro.
Storage
-20°C
Purification Methods
It is an alkaloid neurotoxin which prevents inactivation of Na+ channels. Its solubility in EtOH is ~5%; and it separates as a pale yellow powder from an ethanolic solution on addition of Et2O. It forms nitrate, sulfate and perchlorate salts. [McKinney et al. Anal Biochem 153 33 1986, Beilstein 21 V/13 709.]
References
Merck., Annalen, 95, 200 (1855)
Schmidt, Koppen., Ber., 9, 1115 (1876)
Wright, Luff.,J. Chem. Soc., 35, 421 (1879)
Bosetti., Arch. Pharm., 221,82 (1883)
Blount., Chem. Zeit., 26,334 (1902)
Properties of VERATRIDINE
| Melting point: | 180℃ |
| Boiling point: | 689.74°C (rough estimate) |
| alpha | D20 +8.0° (ethanol) |
| Density | 1.45±0.1 g/cm3 (20 ºC 760 Torr) |
| refractive index | 1.6630 (estimate) |
| storage temp. | -20°C |
| solubility | ethanol: 50 mg/mL |
| form | powder |
| pka | 9.54(at 25℃) |
| color | white to off-white |
| Merck | 13,10016 |
| BRN | 78875 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 2 months. |
| EPA Substance Registry System | Veratridine (71-62-5) |
Safety information for VERATRIDINE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P262:Do not get in eyes, on skin, or on clothing. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for VERATRIDINE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Veratridine CAS 71-62-5View Details
Veratridine CAS 71-62-5View Details
71-62-5 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
