Ki16198
- CAS NO.:355025-13-7
- Empirical Formula: C24H25ClN2O5S
- Molecular Weight: 488.9837
- MDL number: MFCD23704182
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
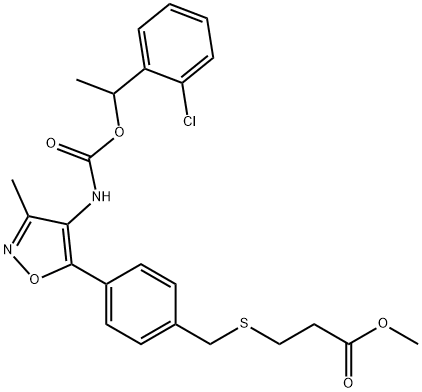
What is Ki16198?
Biological Activity
ki16198 is the methyl ester of ki16425. ki16198, a lpa antagonist, inhibits lpa1- and lpa3-induced inositol phosphate production with the ki of 0.34 μm and 0.93 μm, respectively, shows weaker inhibition for lpa2, no activity at lpa4, lpa5, lpa6[1].lysophosphatidic acid (lpa) is an extracellular signaling lipid involved in regulating cell proliferation, survival, and motility of normal and cancer cells[2].
in vitro
in yapc-pd cancer cell line, ki16198 substantially inhibited lpa1- and lpa3-mediated responses with low potency to lpa2 and no activity to lpa4, lpa5 and lpa6. treatment with ki16198 (10 μm) effectively inhibited migration and invasion responses to lpa in yapc-pd cancer cell line. incubation of ki16198 (10 μm) inhibited the lpa-induced expression of prommp-9 protein and mrna in yapc-pd cells [1].administration of ki16198 (1 μm) inhibited the proliferation of lpa1δ-1 and lpa1δ+-1 cells by about 70% [2].
in vivo
in yapc-pd pancreatic cancer cell-inoculated nude xenograft mouse model, oral administration of ki16198 (2 mg/kg) significantly inhibited tumor weight and remarkably attenuated invasion and metastasis to lung, liver, and brain and decreased the total metastatic node weight in the peritoneal cavity and ascites formation by 50% [1].oral administration of ki16198 (60 mg/kg) significantly inhibited lactate-induced limb lesions in rats [3].
References
[1]. komachi m, sato k, tobo m, et al. orally active lysophosphatidic acid receptor antagonist attenuates pancreatic cancer invasion and metastasis in vivo[j]. cancer science, 2012, 103(6): 1099-1104.
[2]. shano s, hatanaka k, ninose s, et al. a lysophosphatidic acid receptor lacking the pdz-binding domain is constitutively active and stimulates cell proliferation[j]. biochimicaetbiophysicaacta (bba)-molecular cell research, 2008, 1783(5): 748-759.
[3]. kimura t, mogi c, sato k, et al. p2y5/lpa6 attenuates lpa1-mediated ve-cadherin translocation and cell–cell dissociation through g12/13 protein–src–rap1[j]. cardiovascular research, 2011: cvr154.
Properties of Ki16198
| storage temp. | Store at -20°C |
| solubility | insoluble in H2O; ≥2.53 mg/mL in EtOH; ≥24.45 mg/mL in DMSO |
| form | solid |
| color | White to off-white |
Safety information for Ki16198
Computed Descriptors for Ki16198
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium 3-Bromopyrazole (3aR,4R,5R,6aS)-hexahydro-5-Triethyl silyloxy-4-((E)-3-oxo-5-phenylpent-1- enyl)cyclopenta[b]furan-2-one. 1-Chlorocarbonyl-4-piperidinopiperidine 1-Bromo-4-phenyl-2-Butanone 4-Amino-2-fluoro-N-methylbenzamide 1,1'-Carbonyldiimidazole SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTIONRelated products of tetrahydrofuran
You may like
-
![Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
54094-19-8 -
 85-81-4 99%View Details
85-81-4 99%View Details
85-81-4 -
 Cyclopentane carboxxylic acid 3400-45-1 99%View Details
Cyclopentane carboxxylic acid 3400-45-1 99%View Details
3400-45-1 -
![208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 -
 2033-24-1 99%View Details
2033-24-1 99%View Details
2033-24-1 -
 Meldrums acid 2033-24-1 99%View Details
Meldrums acid 2033-24-1 99%View Details
2033-24-1 -
 Cyaclopentane carboxylic acid 99%View Details
Cyaclopentane carboxylic acid 99%View Details
3400-45-1 -
 2-Aminopyridine 504-29-0 99%View Details
2-Aminopyridine 504-29-0 99%View Details
504-29-0