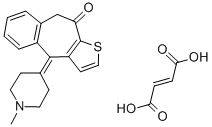
KETOTIFEN
- CAS NO.:34580-13-7
- Empirical Formula: C19H19NOS
- Molecular Weight: 309.43
- MDL number: MFCD00599548
- EINECS: 252-099-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
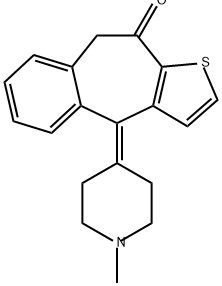
What is KETOTIFEN?
Absorption
Following oral administration, absorption is relatively quick (with a Tmax of ~3 hours) and nearly complete as judged by plasma concentrations and urinary excretion levels - despite this, oral bioavailability is only ~50% due to a significant first-pass effect in the liver.
Toxicity
Oral ingestion of up to 60x the recommended dose has been reported, although no fatal overdoses of ketotifen have been described. Symptoms of ketotifen overdosage may include significant sedation, confusion, disorientation, tachycardia, hypotension, convulsions, hyperexcitability (particularly in children), and/or reversible coma. If ingestion is recent, consider the use of gastric lavage or activated charcoal. Other treatments should be supportive and administered as necessary based on symptoms.
Physostigmine may be useful to mitigate anticholinergic effects, and short-acting barbiturates or benzodiazepines may be used if the patient presents with excitation or convulsions.
Originator
Zaditen,Wander,Switz.,1978
The Uses of KETOTIFEN
Ketotifen is an intermediate in the synthesis of Ketotifen N-Oxide Hydrochloride (K315410), which is a metabolite of Ketotifen (K315100) in humans; formed by human liver microsome. For the free base, see K315115.
The Uses of KETOTIFEN
Anti-asthmatic.
Indications
Administered orally, ketotifen is indicated as an add-on medication in the chronic treatment of mild atopic asthma in children. It is also available as an over-the-counter ophthalmic solution which is indicated for the temporary prevention of itching of the eye due to allergic conjunctivitis.
Background
Ketotifen is a benzocycloheptathiophene derivative with potent antihistaminic and mast cell stabilizing properties. It has a similar structure to some other first-generation antihistamines such as cyproheptadine and azatadine.
Ketotifen was first developed in Switzerland in 1970 by Sandoz Pharmaceuticals and was initially marketed for the treatment of anaphylaxis. In the US, it is now used in an over-the-counter ophthalmic formulation for the treatment of itchy eyes associated with allergies, and in Canada a prescription-only oral formulation is available and indicated as an add-on therapy for children with atopic asthma. In addition, oral ketotifen is used in Mexico and across Europe for the treatment of various allergic symptoms and disorders, including urticaria, mastocytosis, and food allergy.
Definition
ChEBI: Ketotifen is an organic heterotricyclic compound that is 4,9-dihydro-10H-benzo[4,5]cyclohepta[1,2-b]thiophen-10-one which is substituted at position 4 by a 1-methylpiperidin-4-ylidene group. A blocker of histamine H1 receptors with a stabilising action on mast cells, it is used (usually as its hydrogen fumarate salt) for the treatment of asthma, where it may take several weeks to exert its full effect. It has a role as a H1-receptor antagonist and an anti-asthmatic drug. It is an organosulfur heterocyclic compound, an organic heterotricyclic compound, a cyclic ketone, a member of piperidines, an olefinic compound and a tertiary amino compound. It is a conjugate base of a ketotifen(1+).
Manufacturing Process
3.07 g of iodine-activated magnesium shavings are covered with a layer of 25
cc of tetrahydrofuran, and approximately 1/10 of a solution of 17.7 g of 4-
chloro-1-methylpiperidine base in 70 cc of absolute tetrahydrofuran is added.
The Grignard reaction is initiated by the addition of a few drops of 1,2-
dibromoethane. The remaining 4-chloro-1-methylpiperidine solution is then
added dropwise to the magnesium at such a rate that the reaction mixture
boils continuously at reflux without external heating. Boiling at reflux is then
continued for 1 hour. 15.3 g of 10-methoxy-4H-benzo[4,5]cyclohepta[1,2-
b]thiophen-4-one are subsequently added portionwise at 20°C, within 40
minutes, with slight cooling. After stirring at 20°C for 1,5 hours, the reaction
solution is poured on a mixture of 180 g of ice and 20 g of ammonium
chloride. The free base is extracted with chloroform.
The chloroform solution is concentrated and the residue recrystallized from
270 cc of absolute ethanol. The pure 10-methoxy-4-(1-methyl-4-piperidyl)-
4H-benzo[4,5]cyclohepta[1,2-b]thiophen-4-ol base, having a melting point of
194°C to 196°C, is obtained in this manner. Microanalysis corresponds with
the formula C20H23NO2S.
A mixture of 3.4 g of 10-methoxy-4-(1-methyl-4-piperidyl)-4H-benzo[4,5]
cyclohepta [1,2-b]thiophen-4-ol base and 40 cc of 3N hydrochloric acid is kept
in a boiling water bath at 95°C to 100°C for 1 hour. The mixture is
subsequently made alkaline with concentrated caustic soda solution at 20°C
while cooling, and the free base is extracted with chloroform. The chloroform
solution is concentrated, and the residue is recrystallized from ethanol/water
1:1. The pure 4-(1-methyl-4-piperidylidene)-4H-benzo[4,5]cyclohepta [1,2-b]
thiophen-10(9H)-one base, having a melting point of 152°C to 153°C, is
obtained in this manner.
brand name
Zaditor (Novartis).
Therapeutic Function
Anti-asthmatic, Antihistaminic
General Description
Crystals (from ethyl acetate).
Fire Hazard
Flash point data for KETOTIFEN are not available. KETOTIFEN is probably combustible.
Pharmacokinetics
Ketotifen is a non-competitive histamine antagonist and mast cell stabilizer. Administered orally, it functions as a non-bronchodilator antiasthmatic drug by inhibiting the effects of endogenous substances known to be inflammatory mediators. While effects can take 6 to 12 weeks to become apparent, the use of ketotifen has been demonstrated to reduce the frequency, severity, and duration of asthma symptoms, and may allow for a reduction in the use of other asthma therapies.
Clinical Use
Ketotifen is a potent, selective H1 antihistamine that also prevents release of transmitters from mast cells. It is approved in the United States for topical use to prevent itching of the eye because of allergic conjunctivitis, It is used as a systemic antiallergy agent in several countries outside the United States for the treatment of seasonal allergic rhinitis, hay fever, and asthma. Being structurally analogous to the cyproheptadine-like antihistamines, differences in activity of the two enantiomers (atropisomers) has been noted, being approximately six- to seven-fold in ligand displacement and rodent-based assays. Ketotifen has been shown to stabilize mast cells and to inhibit degranulation of eosinophils. Like olopatadine, it has been shown to interact with model membranes, stabilizing them by interaction with phospholipids monolayers.
Metabolism
Ketotifen is extensively metabolized in humans and three distinct metabolites have been detected in human urine. The main metabolite is the N-glucuronide, comprising roughly 50% of urinary drug product, with the N-demethylated nor-ketotifen and the 10-hydroxyl derivative comprising 2% and <1%, respectively. Nor-ketotifen appears to be equally as active as its parent drug, though the clinical relevance of this is unclear given the relatively small proportion in which nor-ketotifen is found in the plasma.
Formation of the N-glucuronide metabolite is carried out by several UGT enzymes, including UGT1A3, UGT1A4, and UGT2B10.
Properties of KETOTIFEN
| Melting point: | 152-153℃ |
| Boiling point: | 488.9±45.0 °C(Predicted) |
| Density | 1.236 |
| storage temp. | Amber Vial, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly) |
| form | Solid |
| pka | 8.84±0.20(Predicted) |
| color | Light Yellow to Orange |
| EPA Substance Registry System | Ketotifen (34580-13-7) |
Safety information for KETOTIFEN
Computed Descriptors for KETOTIFEN
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8