Kaempferol
Synonym(s):3,4ʹ,5,7-Tetrahydroxyflavone;3,4′,5,7-Tetrahydroxyflavone;3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one;Kaempferol - CAS 520-18-3 - Calbiochem;Robigenin
- CAS NO.:520-18-3
- Empirical Formula: C15H10O6
- Molecular Weight: 286.24
- MDL number: MFCD00016938
- EINECS: 208-287-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
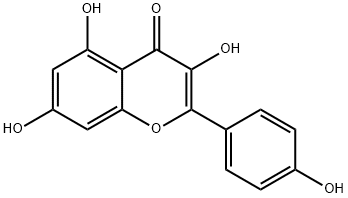
What is Kaempferol?
Description
The flavonoid kaempferol takes its name from one of its sources, aromatic ginger (Kaempferia galanga). It was isolated from forking larkspur (Delphinium consolida?L.) by A. G. Perkin and E. J. Wilkinson in 1902. Since then, it has been found in plants such as grapefruit, tea, broccoli, witch hazel, and apple. Studies on kaempferol show that taking it may lower the risk of contracting pancreatic and lung cancer.
Chemical properties
Yellow Solid
The Uses of Kaempferol
Kaempferol, is used as an inhibitor of Fatty Acid Synthase, Cox-1 activity, and Topo I. It also Induces caspase-9-mediated apoptosis in a variety of cancer cell lines via downregulation of polo-like kinase 1 (PLK1) expression. Exhibits antioxidant activity and attenuates osteoclastic bone reabsorption in vitro. Also blocks EGF-induced histone H3Ser10 phosphorylation in mouse epidermal JB6 C141 cells.
The Uses of Kaempferol
antidepressant, inhibits fatty acid amide hydrolase
The Uses of Kaempferol
Chromogenic reagent for antimony in the low ppm range and for gallium and indium in the sub-ppm range.
What are the applications of Application
Kaempferol is an inhibitor of Fatty Acid Synthase, COX-1 activity, and Topo I
Definition
ChEBI: Kaempferol is a tetrahydroxyflavone in which the four hydroxy groups are located at positions 3, 5, 7 and 4'. Acting as an antioxidant by reducing oxidative stress, it is currently under consideration as a possible cancer treatment. It has a role as an antibacterial agent, a plant metabolite, a human xenobiotic metabolite, a human urinary metabolite, a human blood serum metabolite and a geroprotector. It is a member of flavonols, a 7-hydroxyflavonol and a tetrahydroxyflavone. It is a conjugate acid of a kaempferol oxoanion.
General Description
Kaempferol is a polyphenolic antioxidant abundantly present in vegetables and fruits. It has a diphenylpropane structure. In many plants, it is present as a glycosidic form namely, kaempferol-3-O-glucoside.
Biological Activity
Naturally occurring flavonoid found in Gingko biloba and red wines that activates the mitochondrial Ca 2+ uniporter (EC 50 = 7 μ M). Induces caspase-9-mediated apoptosis in a variety of cancer cell lines via downregulation of polo-like kinase 1 (PLK1) expression. Exhibits antioxidant activity and attenuates osteoclastic bone reabsorption in vitro .
Biochem/physiol Actions
Potent inhibitor of osteoclastic bone resorption. The effect is believed to be attributable to both the antioxidant and estrogenic activities of kaempferol.
Anticancer Research
Kaempferol is one of the secondary metabolites found in some plants, plant-derivedfoods, and traditional medicines. It is a flavonoid compound obtained from someedible plants including grapes, tea, strawberries, broccoli, tomato, cabbage, leek,kale, endive, and beans. It inhibits growth and migration of pancreatic cancer cellsby acting on proto-oncogene tyrosine kinase (Src), ERK1/2, and AKT pathways(Singh et al. 2016a). It is being investigated in pancreatic and lung cancers toevaluate its antiangiogenic, anticancer, and radical scavenging activities. It showsmoderate cytostatic activity in PC3, HeLa, and K562 human cancer cells. It isidentified as aryl hydrocarbon receptor antagonist and acts against ABCG2 (ATP-bindingcassette subfamily G member 2)-mediated multidrug resistance bypreventing the ABCG2 upregulation in esophageal carcinoma. It induces theapoptosis of ovarian cancer cell by activating p53 in intrinsic pathway mechanism.It is an inhibitor of breast cancer resistance protein (BCRP), quinine reductase-2,and a substrate of BCRP (Calderon-Montano et al. 2011; Wang et al. 2012).
Storage
Store at +4°C
Properties of Kaempferol
| Melting point: | 276°C |
| Boiling point: | 348.61°C (rough estimate) |
| Density | 1.2981 (rough estimate) |
| refractive index | 1.4413 (estimate) |
| storage temp. | 2-8°C |
| solubility | ethanol: 20 mg/mL |
| Colour Index | 75640 |
| form | powder |
| pka | 6.34±0.40(Predicted) |
| color | yellow |
| Merck | 14,5274 |
| BRN | 304401 |
| Stability: | Unstable in Solution |
| CAS DataBase Reference | 520-18-3(CAS DataBase Reference) |
| IARC | 3 (Vol. 31, Sup 7) 1987 |
Safety information for Kaempferol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Kaempferol
| InChIKey | IYRMWMYZSQPJKC-UHFFFAOYSA-N |
Kaempferol manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
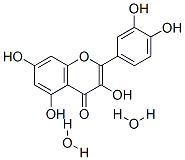
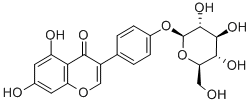
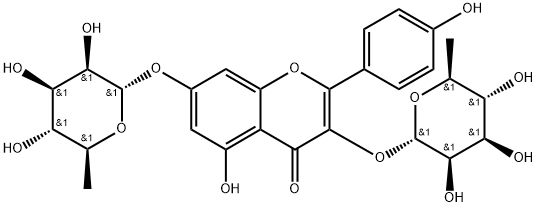
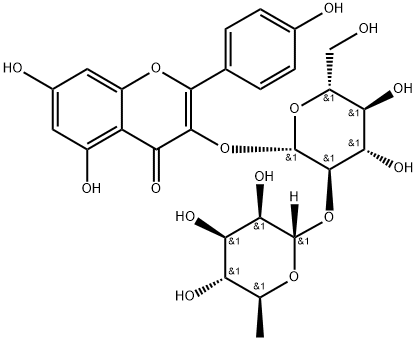
![7-[[2-O-(6-deoxy-alpha-L-mannopyranosyl)-beta-D-glucopyranosyl]oxy]-3,5-dihydroxy-2-(4-hydroxyphenyl)-4H-benzopyran-4-one](https://img.chemicalbook.in/CAS/GIF/17353-03-6.gif)
You may like
-
 Kaempferol 98% supplierView Details
Kaempferol 98% supplierView Details
520-18-3 -
 520-18-3 Kaempferol 98%View Details
520-18-3 Kaempferol 98%View Details
520-18-3 -
 Kaempferol CAS 520-18-3View Details
Kaempferol CAS 520-18-3View Details
520-18-3 -
 Kaempferol 98% CAS 520-18-3View Details
Kaempferol 98% CAS 520-18-3View Details
520-18-3 -
 Kaempferol, ≥98% CAS 520-18-3View Details
Kaempferol, ≥98% CAS 520-18-3View Details
520-18-3 -
 Kaempferol CAS 520-18-3View Details
Kaempferol CAS 520-18-3View Details
520-18-3 -
 Kaempferol CAS 520-18-3View Details
Kaempferol CAS 520-18-3View Details
520-18-3 -
 Kaempferol, ≥98%View Details
Kaempferol, ≥98%View Details
520-18-3
