ARTEMISININ
Synonym(s):3,5,7-Trihydroxy-4′-methoxyflavone;4′-Methoxy-3,5,7-trihydroxyflavone;KF
- CAS NO.:491-54-3
- Empirical Formula: C16H12O6
- Molecular Weight: 300.26
- MDL number: MFCD00016771
- EINECS: 207-738-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 11:41:24
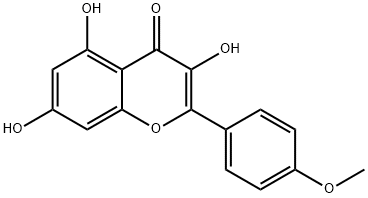
What is ARTEMISININ?
Chemical properties
Yellow powder
The Uses of ARTEMISININ
Kaempferide is a flavonoid that maintains anti-radical and anti-oxidant capabilities, as well as anti-tumour possibilities. Impurity of Icaritin (I163700).
Definition
ChEBI: A monomethoxyflavone that is the 4'-O-methyl derivative of kaempferol.
Biological Activity
the effects of phytoestrogens have been studied in the hypothalamic-pituitary-gonadal axis and various non-gonadal targets. epidemiologic and experimental evidence indicates a protective effect of phytoestrogens also in colorectal cancer. the mechanism through which estrogenic molecules control colorectal cancer tumorigenesis could possibly involve estrogen receptor β, which is the predominantly expressed estrogen receptor subtype in colon mucosa.
in vitro
kaempferide triglycoside proved to inhibit the proliferation of native and estrogen receptor β overexpressing colon cancer cells via a mechanism not mediated by ligand binding dependent estrogen receptor activation. it affected hct8 cell cycle progression through increasing the g0/g1 cell fraction and in estrogen receptor β overexpressing cells increased two antioxidant enzymes [1].
in vivo
the aim of one previous study was to evaluate the effect of kaempferol on tissue lipid peroxidation and antioxidant status in 1,2-dimethyl hydrazine induced colorectal cancer in male wistar rats and to compare its efficacy with irinotecan. this study revealed that kaempferol could be safely used as a chemopreventive agent in colorectal cancer [2].
References
[1] martineti v, tognarini i, azzari c, carbonell sala s, clematis f, dolci m, lanzotti v, tonelli f, brandi ml, curir p. inhibition of in vitro growth and arrest in the g0/g1 phase of hct8 line human colon cancer cells by kaempferide triglycoside from dianthus caryophyllus. phytother res. 2010 sep;24(9):1302-8.
[2] nirmala p, ramanathan m. effect of kaempferol on lipid peroxidation and antioxidant status in 1,2-dimethyl hydrazine induced colorectal carcinoma in rats. eur j pharmacol. 2011 mar 1;654(1):75-9.
Properties of ARTEMISININ
| Melting point: | 156-157 °C(lit.) |
| Boiling point: | 543.8±50.0 °C(Predicted) |
| Density | 1.538 |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | Chloroform (Slightly, Heated), DMSO (Slightly, Heated), Methanol (Slightly, Heat |
| form | neat |
| pka | 6.32±0.40(Predicted) |
| form | Solid |
| color | Yellow |
| BRN | 305378 |
| CAS DataBase Reference | 491-54-3 |
Safety information for ARTEMISININ
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for ARTEMISININ
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Kaempferide CAS 491-54-3View Details
Kaempferide CAS 491-54-3View Details
491-54-3 -
 Kaempferide 95% CAS 491-54-3View Details
Kaempferide 95% CAS 491-54-3View Details
491-54-3 -
 Kaempferide CAS 491-54-3View Details
Kaempferide CAS 491-54-3View Details
491-54-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1