Josamycin
Synonym(s):Josamycin;Leucomycin A3
- CAS NO.:16846-24-5
- Empirical Formula: C42H69NO15
- Molecular Weight: 827.99
- MDL number: MFCD00210320
- EINECS: 240-871-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
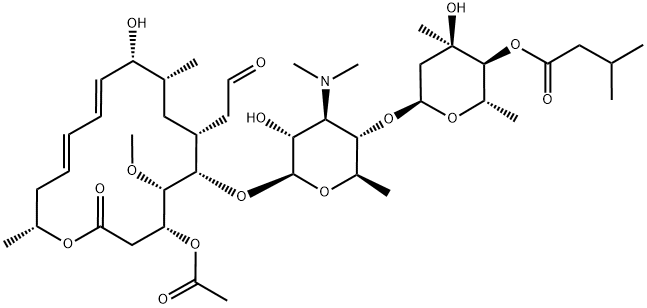
What is Josamycin?
Chemical properties
Josamycin is a macrolide substance having antibacterial activity produced by the growth of Streptomyces narboensis var. josamyceticus.
Josamycin appears as white to yellowish white powder, slightly hygroscopic. Josamycin is very soluble in methanol or in ethanol, and very slightly soluble in water.
Originator
Josalid,Biochemie
The Uses of Josamycin
As a 16-membered ring macrolide antibiotic with antimicrobial activity against a wide range of pathogens. Josamycin can be particularly used in the treatment of Mycoplasma infection.
Josamycin is used to study the modification of phagocytosis and cytokine production by macrolide antibiotics, immunomodulatory effects, the suppression of matrix metalloproteinase production as well as study bacteria protein synthesis at the level of the the 23S rRNA, 50S ribosomal subunit.
The Uses of Josamycin
Josamycin, is used as a macrolide antibiotic effective against a variety of pathogens.
What are the applications of Application
Josamycin is a macrolide antibiotic effective against a variety of pathogens
Definition
ChEBI: A macrolide antibiotic produced by certain strains of Streptomyces narbonensis var. josamyceticus.
Manufacturing Process
100 ml of a culture medium consisting of water containing 1.5% soybean
meal, 1% starch, 1% glucose, 0.3% sodium chloride, 0.1% dipotassium
hydrogen phosphate, and 0.05% magnesium sulfate was placed in a 500 ml
flask and sterilized for 20 min at 120°C. After cooling, the culture medium
was inoculated with strain A 205-P2 Streptomyces narbonensis var. josatny
ceticus, and the strain was subjected to shaking culture at 27°-29°C and at
130 strokes per min and 8 cm amplitude. After 3 days of culture, the culture
fluids in such 100 flasks were combined together and filtered to give 8700 ml
of culture filtrate. The pH of the filtrate was 6.4 and showed an inhibition zone
of 25 mm. to Bacillus subtilis (PCI 219 strain). The filtrate was extracted with
8700 ml of ethyl acetate. The extract (7300 ml) thus obtained was
concentrated to 730 ml under vacuum at temperatures lower than 50°C, 360
ml of water added, and then concentrated hydrochloric acid added to ad just
the pH to 2.0, whereby josamycin was transferred to the aqueous layer. After
adjusting the pH of the aqueous layer to 7.5 by the addition of 0.1 N sodium
hydroxide, josamycin was extracted with 180 ml of ethyl acetate.
Josamycin was then transferred to 90 ml of an aqueous solution at pH 2.0 and
extracted again with 45 ml of ethyl acetate as above process. Ethyl acetate
solution thus obtained was evaporated under reduced pressure to give a
solidified product, which was dissolved in 5 ml of benzene to remove
impurities and the product, solidified from the benzene solution by
evaporating under reduced pressure, was dissolved in a small amount of ethyl
acetate and subjected to an alumina chromatography. That is, Brockman
alumina (Merck) was treated with hydrochloric acid, sufficiently rinsed with
water, and activated by heating for 5 h at 150°C.
50.0 g of thus treated alumina was filled in a glass tube of 1.6 cm in diameter
by using ethyl acetate. The above prepared ethyl acetate solution was added
to the alumina column and the product was eluted with 200 ml of ethyl
acetate. The eluate thus obtained was concentrated under reduced pressure
and the solid product thus obtained was dissolved in 5 ml of benzene and 50
ml of n-hexane added to give 0.18 g of amorphous josamycin having a purity
of above 90%.
Therapeutic Function
Antibiotic
Pharmaceutical Applications
A naturally occurring antibiotic produced by Streptomyces narbonensis var. josamyceticus and belonging to the leucomycin group of macrolides. It is formulated for oral administration.
Many Gram-positive and Gram-negative anaerobes are susceptible, including Peptostreptococcus spp., Propionibacterium spp., Eubacterium spp. and Bacteroides spp.
After a single 1 g oral dose, a peak serum concentration of 2.74 mg/L was achieved 0.75 h after dosing. The AUC was 4.2 mg.h/L, and the apparent elimination half-life 1.5 h. Several inactive metabolites could be detected. It penetrates into saliva, tears and sweat, and achieves high levels in bile and lungs. It is mostly metabolized and excreted in the bile in an inactive form. Less than 20% of the dose appears in the urine, producing levels of around 50 mg/L.
The drug is generally well tolerated, producing only mild gastrointestinal disturbance. Its uses are similar to those of erythromycin. It is of limited availability.
Biological Activity
Josamycin inhibits bacterial protein biosynthesis by inhibiting peptidyltransferase and ribosomal translocation, and depleting the intracellular pools of aminoacyl-tRNAs available for protein synthesis by drop-off and incomplete peptidyl-tRNA hydrolase activity. It slows down formation of the first peptide bond of a nascent peptide in an amino acid-dependent way and inhibits formation of the second or third peptide bond.
Properties of Josamycin
| Melting point: | 131.5℃ |
| Boiling point: | 763.27°C (rough estimate) |
| alpha | D25 -70° (c = 1 in ethanol) |
| Density | 1.1547 (rough estimate) |
| refractive index | 1.6220 (estimate) |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | Soluble in ethanol |
| pka | 7.1 (40% aq methanol) |
| form | powder |
| color | white to slightly yellow |
| Merck | 13,5286 |
| Stability: | Hygroscopic |
Safety information for Josamycin
Computed Descriptors for Josamycin
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Josamycin CAS 16846-24-5View Details
Josamycin CAS 16846-24-5View Details
16846-24-5 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1