Isonicotinic acid
Synonym(s):INA;4-Pyridinecarboxylic acid;Pyridine-4-carboxylic acid;4-Picolinic acid;66 kDa neurofilament protein
- CAS NO.:55-22-1
- Empirical Formula: C6H5NO2
- Molecular Weight: 123.11
- MDL number: MFCD00006429
- EINECS: 200-228-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 14:18:24
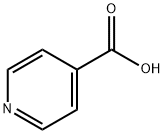
What is Isonicotinic acid?
Chemical properties
isonicotinic acid is also known as pyridine-4-carboxylic acid. white to light yellow crystal powder or white needle crystals. odorless, sublimable. molecular weight 123.11. melting point 319°C . slightly soluble in cold water, soluble in hot water, insoluble in alcohol, benzene and ether. isonicotinic acid is an amphoteric compound that is soluble in both acids and bases. soluble in hot water and ethanol, slightly soluble in cold water and ether. stable to heat and oxidation.
The Uses of Isonicotinic acid
Isonicotinic acid is a metabolite of isoniazid. It is an isomer of nicotinic acid. The chronic toxicity of isonicotinic acid is slightly higher than that of isonicotinic acid hydrazide ( INH ) .
Isonicotinic acid is a moderately basic compound (based on its pKa). Isonicotinic acid is an organic compound with a carboxyl group on a pyridine ring. The carboxyl group for isonicotinic acid is on the 4-position instead of the 3-position for nicotinic acid. It is an isomer of nicotinic acid. Isonicotinic acid is a metabolite of isoniazid.
Isonicotinic acid considered to be inactive isomer of nicotinic acid. Isonicotinic acid is a metabolite of pyridine-4-carboxy hydrazide (isonicotinyl hydrazide; isoniazid) a front-line weapon in the battle against tuberculosis. Isonicotinic acid and its derivatives are used in manufacturing pharmaceuticals and agrochemicals.
Used in the biological study of differentiation induced by nicotinic acid, nicotinamide and isonicotinic acid in human leukemia cell lines
What are the applications of Application
Isonicotinic acid is a useful isomer of nicotinic acid
Definition
ChEBI: Isonicotinic acid is a pyridinemonocarboxylic acid in which the carboxy group is at position 4 of the pyridine ring. It has a role as a human metabolite and an algal metabolite. It is a conjugate acid of an isonicotinate.
Preparation
Isonicotinic acid is synthesized by continuous oxidation with 4-methylpyridine as raw material and vanadium pentoxide as catalyst. The purity of the industrial product isonicotinic acid is more than 95%, and the yield of the above method is 70-75%. The consumption of 4-methylpyridine per ton of product is 1070kg.
What are the applications of Application
Isonicotinic acid (IN) can be used as:
An organocatalyst in the one pot four component condensation reaction, to synthesize pyranopyrazoles based heterocyclic compounds.
An organic ligand for the preparation of copper(I) halide coordination polymer [CuBr(IN)]n, by hydrothermal method.
As a starting material for the synthesis of cation-dimers with potent antimalarial activity.
Purification Methods
Crystallise the acid repeatedly from water and dry it under vacuum at 110o or sublime it at 260o/15mm (m 319o). [Beilstein 22 III/IV 518, 22/2 V 188.]
Properties of Isonicotinic acid
| Melting point: | ≥300 °C (lit.) |
| Boiling point: | 260 °C (15 mmHg) |
| Density | 1,47g/cm |
| refractive index | 1.5423 (estimate) |
| Flash point: | 260°C/15mm |
| storage temp. | no restrictions. |
| solubility | 6g/l |
| form | powder |
| pka | 4.96(at 25℃) |
| color | yellow to tan |
| PH | 3-4 (6g/l, H2O, 20℃)(saturated solution) |
| Water Solubility | 5.2 g/L (20 ºC) |
| Merck | 14,5187 |
| BRN | 109599 |
| CAS DataBase Reference | 55-22-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Isonicotinic acid(55-22-1) |
| EPA Substance Registry System | Isonicotinic acid (55-22-1) |
Safety information for Isonicotinic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Isonicotinic acid
| InChIKey | TWBYWOBDOCUKOW-UHFFFAOYSA-N |
Isonicotinic acid manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Isonicotinic acid 98%View Details
Isonicotinic acid 98%View Details
55-22-1 -
 Isonicotinic acid CAS 55-22-1View Details
Isonicotinic acid CAS 55-22-1View Details
55-22-1 -
 Isonicotinic Acid CASView Details
Isonicotinic Acid CASView Details -
 Isonicotinic acid 98.00% CAS 55-22-1View Details
Isonicotinic acid 98.00% CAS 55-22-1View Details
55-22-1 -
 isonicotinic acid 98% CAS 55-22-1View Details
isonicotinic acid 98% CAS 55-22-1View Details
55-22-1 -
 Pyridine-4-Carboxylic Acid, 25kgView Details
Pyridine-4-Carboxylic Acid, 25kgView Details
55-22-1 -
 ISONICOTINIC ACID (55-22-1)View Details
ISONICOTINIC ACID (55-22-1)View Details
55-22-1 -
 Iso Nicotinic Acid, 50kgView Details
Iso Nicotinic Acid, 50kgView Details
55-22-1
