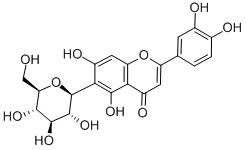
Isoliquiritigenin
Synonym(s):(E)-1-(2,4-Dihydroxyphenyl)-3-(4-hydroxyphenyl)-2-propen-1-one;4,2′,4′-Trihydroxychalcone
- CAS NO.:961-29-5
- Empirical Formula: C15H12O4
- Molecular Weight: 256.25
- MDL number: MFCD00075907
- EINECS: 607-884-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-29 14:36:51
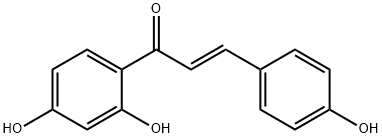
What is Isoliquiritigenin?
Chemical properties
Yellow solid
The Uses of Isoliquiritigenin
Isoliquiritigenin (ISL) is a flavonoid found in licorice root and several other plants that displays antioxidant, anti-inflammatory, and antitumor activities as well as hepatoprotection against steatosis-induced oxidative stress. ISL induces quinone reduc
The Uses of Isoliquiritigenin
aldose reductase inhibitor, antineoplastic, antiinflammatory
The Uses of Isoliquiritigenin
Isoliquiritigenin (ISL) is a flavonoid found in licorice root and several other plants that displays antioxidant, anti-inflammatory, and antitumor activities as well as hepatoprotection against steatosis-induced oxidative stress. ISL induces quinone reductase-1, a phase II enzyme that deactivates radicals and electrophiles, with a concentration required to double activity (CD) value of 1.8 μM in murine hepatoma cells.
What are the applications of Application
Isoliquiritigenin is a GCS (soluble guanylyl cyclase) activator
Definition
ChEBI: Isoliquiritigenin is a member of the class of chalcones that is trans-chalcone hydroxylated at C-2', -4 and -4'. It has a role as an EC 1.14.18.1 (tyrosinase) inhibitor, a biological pigment, a NMDA receptor antagonist, a GABA modulator, a metabolite, an antineoplastic agent and a geroprotector. It is functionally related to a trans-chalcone. It is a conjugate acid of an isoliquiritigenin(1-).
General Description
Isoliquiritigenin is an aromatic ketone and belongs to the chalcone group of compound. It is derived from licorice and is a component in medicine and food.
Biochem/physiol Actions
Isoliquiritigenin is a soluble guanylyl cyclase activator and possesses antitumor activity. It also possesses antioxidant, antiplatelet and estrogenic properties.
Anticancer Research
It is extracted from Dipteryx odorata seeds, found to be active in induction of quininereductase, and thus prevents chemical carcinogenesis. In a dose- and time-dependentmanner, it significantly inhibits the proliferation of prostate cancer cells,and this isoliquiritigenin-induced cell cycle arrest and antiproliferative effects maybe manifested by growth arrest- and DNA damage-inducible gene 153 (GADD153).It induces apoptosis in prostate cancer cells through mitochondrial apoptosispathway in which mitochondrial membrane potential is disrupted, cytochrome-cand Smac/Diablo are released, and caspase-9 is activated. It is reported to enhancethe expression of universal inhibitor of cyclin-dependent kinases, p21CIP1/WAF1, in adose- and time-dependent manner in A549 human lung cancer cells (Kanazawaet al. 2003; Ii et al. 2004; Balunas and Kinghorn 2005; Jung et al. 2006).
storage
+4°C
Properties of Isoliquiritigenin
| Melting point: | 206-210°C |
| Boiling point: | 504.0±42.0 °C(Predicted) |
| Density | 1.384±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | H2O: <0.1 mg/mL |
| form | powder |
| pka | 7.50±0.35(Predicted) |
| color | yellow to orange |
| Sensitive | Light Sensitive & Hygroscopic |
| BRN | 1914296 |
| CAS DataBase Reference | 961-29-5(CAS DataBase Reference) |
Safety information for Isoliquiritigenin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Isoliquiritigenin
| InChIKey | DXDRHHKMWQZJHT-FPYGCLRLSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Isoliquiritigenin 98% (HPLC) CAS 961-29-5View Details
Isoliquiritigenin 98% (HPLC) CAS 961-29-5View Details
961-29-5 -
 Isoliquiritigenin CAS 961-29-5View Details
Isoliquiritigenin CAS 961-29-5View Details
961-29-5 -
 Isoliquiritigenin CAS 961-29-5View Details
Isoliquiritigenin CAS 961-29-5View Details
961-29-5 -
 Isoliquiritigenin CAS 961-29-5View Details
Isoliquiritigenin CAS 961-29-5View Details
961-29-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1