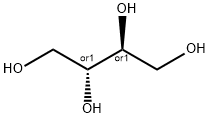
Isomalt
Synonym(s):6-O-α-D -Glucopyranosyl-D -glucitol mixed with 1-O-α-D -glucopyranosyl-D -mannitol;6-O-α-D -Glucopyranosyl-D -glucitol;Isomalt
- CAS NO.:64519-82-0
- Empirical Formula: C12H24O11
- Molecular Weight: 344.31
- MDL number: MFCD00190708
- EINECS: 908-700-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
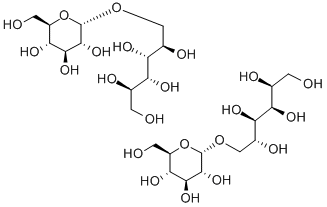
What is Isomalt?
Description
Isomalt is an equimolar mixture of oc-D-glucopyranosyl-1,6-
D-sorbitol (GPS) and α-D-glucopyranosyl-l,l-D-mannitol (GPM).
The production process of isomalt involves two essential steps. In
the first step, the 1,2 -glycosidic linkage between the glucose and
the fructose moiety of sucrose is rearranged by an immobilized
enzyme system to a 1,6 -glycosidic linkage, which is more stable
and characteristic for the cross linkage in the amylopectin fraction
of native starch. In the second step, the rearranged sucrose molecule, i.e., isomaltulose, is hydrogenated to the corresponding sugar
alcohols. Isomalt is commercially available as a dry, white crystalline powder which is about half as sweet as sucrose.
In vitro studies with intestinal disaccharidases from mammals
demonstrated that isomalt is cleaved much more slowly than
sucrose or maltose. A slight inhibitory effect on maltose hydrolysis
and on the active transport on glucose has also been observed.
Ingested isomalt is partly hydrolyzed in the small intestine and
slowly absorbed in the form of glucose, sorbitol, and mannitol.
The intact portion of isomalt, as well as some unabsorbed sorbitol
and mannitol, reaches the distal parts of the gut where these
products are fermented to volatile fatty acids.
Chemical properties
White or almost white powder or granules.
Chemical properties
Isomalt is a more or less equimolar mixture of 1-O-a-D-glucopyranosy-D-mannitol-
dihydrate and 6-O-a-D-glucopyranosyl-D-sorbitol. Different production
conditions, however, allow variations in the ratio of the two products. The solubility
in water is about 24.5 % (w/w) at room temperature, but varies with the
composition and increases with increasing temperature. In addition to the dry
isomalt, a syrup is available.
Isomalt is, depending on the concentration, approximately 45–60 % as
sweet as sucrose, stable under normal processing conditions of foods, and
noncariogenic.
In the European Union, isomalt is approved as E 953 for a large number of food
applications. It is GRAS in the United States and also approved in many other
countries.
Owing to its low glycemic index, isomaltulose, an intermediate of the production,
has found increasing interest as a food ingredient in recent years.
Chemical properties
Isomalt is a sugar alcohol (polyol) that occurs as a white or almost white powder or granular or crystalline substance. It has a pleasant sugarlike taste with a mild sweetness approximately 50–60% of that of sucrose.
History
It is claimed that isomalt is odorless, white, crystalline, and sweet tasting without the accompanying taste or aftertaste. Sweetening power is from 0.45 to 0.6 that of sucrose. A synergistic effect is achieved when isomalt is combined with other artificial sweeteners and sugar substitutes. Principal applications are in confections, pan-coated goods, and chewing gum. The substance was approved for use in most European countries in 1985. Classification of isomalt as a GRAS substance was petitioned in the United States. (GRAS = generally regarded as safe.)
The Uses of Isomalt
analgesic, NSAID
Production Methods
Isomalt is produced from food-grade sucrose in a two-stage process. Beet sugar is converted by enzymatic transglucosidation into the reducing disaccharide isomaltulose. This undergoes catalytical hydrogenation to produce isomalt.
Production Methods
Developed in Germany, isomalt is described as an energy-reduced bulk sweetener and marketed in Europe under the tradename Palatinit? mark. The compound is produced from sucrose in a two-step process. In the first step, the easily hydrolyzable 1-2 glucoside linkage between the glucose and fructose moieties of sucrose are catalyzed by immobilized enzymes to produce isomaltulose, Palatinos mark After crystallization, the isomaltulose is hydrogenated in a neutral aqueous solution using a nickel catalyst.
Definition
ChEBI: (2xi)-6-O-alpha-D-glucopyranosyl-D-arabino-hexitol is a glycosyl alditol consisting of alpha-D-glucopyranose and (2xi)-D-arabino-hexitol residues joined in sequence by a (1->1) glycosidic bond. It is functionally related to an alpha-D-glucose.
Biotechnological Production
For the production of isomalt sucrose is converted to isomaltulose which is then
hydrogenated to yield a mixture of the two components of isomalt. Although
the production of isomalt itself from isomaltulose is a chemical hydrogenation,
transformation of sucrose into isomaltulose requires enzymatic transformation.
The enzyme sucrosemutase is sensitive to glutaraldehyde, therefore crosslinking
is not possible. For industrial use it is, however, not necessary to isolate the
enzyme, as immobilized cells of the organism can be used. Addition of sodium
alginate to the cultivated cells and subsequent addition of calcium acetate
immobilizes the cells. This allows for the use of the cells in a bed reactor, and also
facilitates the separation of the product from the reaction mixture.
The long-term stability of the immobilized organism is high and can exceed
5,000 h, even if high sucrose concentrations of 550 g/L are used. The yields are
about 80–85 % with 9–11 % of trehalulose and small quantities of other saccharides
as by-products.
Prior to hydrogenation, free sucrose has to be removed. This is carried out by
nonviable cells of Saccharomyces cerevisiae. Remaining by-products of the
reaction are converted to the respective sugar alcohols.
Although the hydrogenation of isomaltulose theoretically should yield an
equimolar mixture of the two constituents of isomalt, the share of each component
may vary between 43–57 % depending on the conditions of hydrogenation.
An alternative possibility is the direct cultivation of suitable microorganisms
such as P. rubrum on sucrose-containing juices obtained during the production of beet and cane sugar. It is claimed that glucose and fructose produced during the
transformation are consumed by the microorganisms which results in lower
amounts of by-products.
Pharmaceutical Applications
Isomalt is a noncariogenic excipient used in a variety of
pharmaceutical preparations including tablets or capsules, coatings,
sachets, and suspensions, and in effervescent tablets. It can also be
used in direct compression and wet granulation.
In buccal applications such as chewable tablets it is commonly
used because of its negligible negative heat of solution, mild
sweetness, and ‘mouth feel’. It is also used widely in lozenges,
sugar-free chewing gum, and hard-boiled candies, and as a
sweetening agent in confectionery for diabetics.
Safety
Isomalt is used in oral pharmaceutical formulations, confectionery,
and food products. It is generally regarded as a nontoxic,
nonallergenic, and nonirritant material.
Toxicological and metabolic studies on isomalt have been
summarized in a WHO report prepared by the FAO/WHO Expert
Committee (JECFA), resulting in an acceptable daily intake of ‘not
specified’.
The glycosidic linkage between the mannitol or sorbitol moiety
and the glucose moiety is very stable, limiting the hydrolysis and
absorption of isomalt in the small intestine. There is no significant
increase in the blood glucose level after oral intake, and glycemic
response is very low, making isomalt suitable for diabetics. The
majority of isomalt is fermented in the large intestine. In general,
isomalt is tolerated very well, although excessive consumption may
result in laxative effects.
Isomalt is not fermented by bacteria present in the mouth;
therefore no significant amount of organic acid is produced that
attacks tooth enamel.
Storage
Isomalt has very good thermal and chemical stability. When it is
melted, no changes in the molecular structure are observed. It exhibits considerable resistance to acids and microbial influences.
Isomalt is non-hygroscopic, and at 25°C does not significantly
absorb additional water up to a relative humidity (RH) of 85%;
paracetamol (acetaminophen) tablets based on isomalt were stored
for 6 months at 85% RH at 20°C and retained their physical
aspect.
If stored under normal ambient conditions, isomalt is chemically
stable for many years. When it is stored in an unopened container at
20°C and 60% RH, a re-evaluation after 3 years is recommended.
Regulatory Status
GRAS listed. Accepted as a food additive in Europe.
Properties of Isomalt
| Boiling point: | 788.5±60.0 °C(Predicted) |
| Density | 1.69±0.1 g/cm3(Predicted) |
| vapor pressure | 0-0Pa at 20-50℃ |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Freely soluble in water, practically insoluble in anhydrous ethanol. |
| form | neat |
| pka | 12.89±0.70(Predicted) |
| form | Solid |
| color | White to off-white |
| Odor | at 100.00?%. odorless |
| Water Solubility | Water: 250 mg/mL (726.09 mM) |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 64519-82-0(CAS DataBase Reference) |
Safety information for Isomalt
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Isomalt
| InChIKey | RWJWQKXVEITNKS-JSOWRAQNSA-N |
Isomalt manufacturer
Clickchem Research LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![(2R,3S,4R,5R)-2,3,4,5-tetrahydroxy-6-[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-hexanoic acid](https://img.chemicalbook.in/CAS/GIF/534-74-7.gif)
You may like
-
 Isomalt 97% CAS 64519-82-0View Details
Isomalt 97% CAS 64519-82-0View Details
64519-82-0 -
 Isomalt CAS 64519-82-0View Details
Isomalt CAS 64519-82-0View Details
64519-82-0 -
 Isomalt CAS 64519-82-0View Details
Isomalt CAS 64519-82-0View Details
64519-82-0 -
 Isomalt CAS 64519-82-0View Details
Isomalt CAS 64519-82-0View Details
64519-82-0 -
 64519-82-0 90 % AboveView Details
64519-82-0 90 % AboveView Details
64519-82-0 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
