Methyl isothiocyanate
Synonym(s):High affinity nerve growth factor receptor;MTC;NTRK1;TRK;TRKA
- CAS NO.:556-61-6
- Empirical Formula: C2H3NS
- Molecular Weight: 73.12
- MDL number: MFCD00004818
- EINECS: 209-132-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
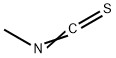
What is Methyl isothiocyanate?
Description
Methyl iso thio cyanate is the organo sulfur compound with the formula CH3N = C = S . This low melting colorless solid is a powerful lachrymator. As a precursor to a variety of valuable bioactive compounds, it is the most important organic iso thio cyanate in industry.
Chemical properties
colourless solid
Chemical properties
Methyl isothiocyanate is a crystalline solid. Horseradish, acrid odor.
Chemical properties
Colorless to tan liquid; pungent, penetrating, mustard-like odor.
Occurrence
Reported found in horseradish.
The Uses of Methyl isothiocyanate
Usually used to study the effect of pesticide Metam (methyl isothiocyanate is its active ingredient) in the streamside microbial communities of the upper Sacramento river
The Uses of Methyl isothiocyanate
Pesticide; soil fumigant.
The Uses of Methyl isothiocyanate
Pesticide and soil fumigant used to control insects, soil fungi, nematodes.
Definition
ChEBI: An isothiocyanate having a methyl group attached to the nitrogen.
What are the applications of Application
Solutions of MITC are used in agriculture as soil fumigants, mainly for protection against fungi and nematodes.
MITC is a building block for the synthesis of 1,3,4 - thiadiazoles, which are heterocyclic compounds used as herbicides. Commercial products include "Spike", "Ustilan," and "Erbotan." Well known pharmaceuticals prepared using MITC include Zantac and Tagamet.
Reactions
A characteristic reaction is with amines to give methyl thioureas:
CH3NCS + R2NH → R2NC(S)NHCH3
Other nucleophiles add similarly.
Aroma threshold values
Very high strength odor; recommend smelling in a 0.01% solution or less
General Description
A colorless liquid with a sharp odor. Lethal by inhalation of even small quantities of vapor. Does not have odor warning characteristics at low concentrations. Do not rely on the sense of smell to warn about the presence of vapors. Denser than water. Flash point below 20°F. May cause tearing and irritate the eyes, skin, nose and throat.
Air & Water Reactions
Highly flammable. Methyl isothiocyanate reacts with water to form carbon dioxide and methylamine gases.
Reactivity Profile
Isocyanates and thioisocyanates, such as Methyl isothiocyanate, are incompatible with many classes of compounds, reacting exothermically to release toxic gases. Reactions with amines, aldehydes, alcohols, alkali metals, ketones, mercaptans, strong oxidizers, hydrides, phenols, and peroxides can cause vigorous releases of heat. Acids and bases initiate polymerization reactions in these materials. Some isocyanates react with water to form amines and liberate carbon dioxide. Polyurethanes are formed by the condensation reaction of diisocyanates with, for example, ethyl glycol.
Hazard
Toxic by ingestion, strong irritant to eyesand skin.
Health Hazard
Very toxic; probable human oral lethal dose is 50-500 mg/kg, or between 1 teaspoonful and 1 oz. for a 70 kg (150 lb.) person. Highly irritating to skin, mucous membrances, and eyes. Human oral minimum lethal dose: approximately 1 g/kg.
Fire Hazard
(Non-Specific -- Pesticide, Solid, n.o.s.) Methyl isothiocyanate may burn, but does not ignite readily. Fire may produce irritating or poisonous gases. When heated Methyl isothiocyanate emits very dangerous cyanides and sulfur compounds. Do not store below -4F or at elevated temperatures. Keep away from sparks.
Flammability and Explosibility
Non flammable
Safety
MITC is a dangerous lachrymator as well as being poisonous.
Synthesis
It is prepared industrially by two routes. Annual production in 1993 was estimated to be 4M kg. The main method involves the thermal rearrangement of methyl thio cyanate :
CH3S - C ≡ N → CH3N = C = S
It is also prepared via with the reaction of methylamine with carbon disulfide followed by oxidation of the resulting dithio carbamate with hydrogen peroxide. A related method is useful to prepare this compound in the laboratory.
MITC forms naturally upon the enzymatic degradation of gluco capparin , a modified sugar found in capers.
Potential Exposure
It is used as a soil fumigant. A mixture of methyl isothiocyanate and chlorinated C-3 hydrocarbons is used as a soil fumigant for control of weeds, fungi, insects, and nematodes.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at leastFirst Aid: If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifing upper and lower lids. Seek med-ical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, includ-ing resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medi-cal attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24- -48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a cortico-steroid spray.
Environmental Fate
Soil. Though no products were reported, the reported half-life in soil is <14 days
(Worthing and Hance, 1991).
Chemical/Physical. Emits toxic fumes of nitrogen and sulfur oxides when heated to
decomposition (Sax and Lewis, 1987).
Metabolic pathway
Methyl isothiocyanate is slowly decomposed in pure water, forming methylamine. It is metabolised in rats to the mercapturate via the glutathione conjugate which serves as a potential source of methyl isothiocyanate. Methyl isothiocyanate is reactive with cellular thiols and amines and its toxicity is associated with these reactions.
Storage
(1) Color Code—Red: Flammability Hazard: Storein a flammable liquid storage area or approved cabinetaway from ignition sources and corrosive and reactivematerials. (2) Color Code—Blue: Health Hazard/Poison:Store in a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area. Sources of ignition, such as smoking andopen flames, are prohibited where methyl isothiocyanate isused, handled, or stored in a manner that could create apotential fire or explosion hazard. Metal containers involving the transfer of 5 gallons or more of this chemical shouldbe grounded and bonded. Drums must be equipped withself-closing valves, pressure vacuum bungs, and flamearresters. Use only nonsparking tools and equipment, especially when opening and closing containers of this chemical. Wherever this chemical is used, handled, manufactured,or stored, use explosion-proof electrical equipment andfittings.
Shipping
UN2477 Methyl isothiocyanate, Hazard class: 6.1; Labels: 6.1-Poison Inhalation Hazard, 3-Flammable liquid, Inhalation Hazard Zone B.
Degradation
Methyl isothiocyanate is unstable and reactive. It is rapidly hydrolysed by
alkalis but more slowly in acidic or neutral solutions. DT50 values for
hydrolysis were 85,490 and 110 hours at pH 5,7 and 9, respectively. It is
sensitive to oxgen and to light (PM). The relatively slow hydrolysis of
methyl isothiocyanate in pure water can be accelerated by adding high
concentrations of acid. Thiocarbamic acid is formed that in turn
decomposes rapidly to protonated methylamine. Addition of water to the
isothiocyanate N=C bond via a mechanism involving synchronous nucleophilic
attack at carbon and proton transfer to nitrogen with a cyclic transition
state is proposed. Methyl isothiocyanate is 107 times less susceptible
to acid catalysis in water than its O-analogue (Joseph et al., 1992). It will
also combine with various essential nucleophilic centres in biological
systems. For example, it reacts with cysteine in vitro, forming a dithiocarbamate
derivative in solutions of pH greater than 6 (Goksoyr, 1964).
The fungicide is photolysed in the gas phase with a half life of 10 hours
under irradiation from a xenon arc lamp and slightly more than one day
for late summer sunlight in Nevada, USA. The relatively rapid photolysis
of methyl isothiocyanate had not previously been observed in other
experiments in aqueous solutions where rates were 20 times slower.
Products of photolysis were methyl isocyanide (2), sulfur dioxide, hydrogen
sulfide, N-methylformamide (3), methylamine and carbonyl sulfide.
Methyl isocyanide (2) was initially the main product and it yielded
methyl isocyanate (4) (Geddes et al., 1995).
Toxicity evaluation
MITC stimulates chemesthesis, the activation of receptors associated with sensations of pain, touch, or thermal perception, through activation of transient receptor potential (TRPA1) ion channels. Isothiocyanate molecules have an electrophilic carbon atom that reacts with nucleophilic components, such as cysteine residues in the TRPA1 channels that are highly sensitive and serve as a warning mechanism to prevent tissue damage. MITC can form a reversible covalent bond with receptors to stimulate a reaction instead of acting directly through tissue damage. MITC is less potent than other isothiocyanates, such as allyl isothiocyanate (i.e., the active component of horseradish, wasabi, and mustard). At low concentrations, endogenous nucleophiles (e.g., GSH) may neutralize the electrophilic carbon and, therefore, prevent damage, but as concentrations increase their effectiveness decreases. Although the mode of action of MITC for systemic toxicity is not known, MITC is proposed to react with, and inactivate, the sulfhydryl group of essential enzymes in living organisms.
Incompatibilities
Dust may form explosive mixture with air. Reacts with water, releasing carbon dioxide and methylamine gases. Unstable and reactive; sensitive to oxygen and to light. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, alcohols, strong bases, amines, water, heat and cold. Attacks iron, zinc and other metals
Waste Disposal
In accordance with 40CFR 165 recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office
Properties of Methyl isothiocyanate
| Melting point: | 30-34 °C(lit.) |
| Boiling point: | 117-118 °C(lit.) |
| Density | 1.069 g/mL at 25 °C(lit.) |
| vapor pressure | 21 mm Hg ( 20 °C) |
| refractive index | nD37 1.5258 |
| FEMA | 4426 | METHYL ISOTHIOCYANATE |
| Flash point: | 90 °F |
| storage temp. | 2-8°C |
| solubility | H2O: slightly soluble |
| form | Low Melting Solid |
| color | White to pale yellow |
| Odor | at 0.01 % in propylene glycol. pungent mustard horseradish |
| Water Solubility | 7.6 g/L |
| Sensitive | Moisture Sensitive |
| Merck | 14,6092 |
| JECFA Number | 1884 |
| BRN | 605319 |
| Stability: | Stable. Incompatible with strong oxidizing agents, alcohols, strong bases, amines. |
| CAS DataBase Reference | 556-61-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Methane, isothiocyanato-(556-61-6) |
| EPA Substance Registry System | Methyl isothiocyanate (556-61-6) |
Safety information for Methyl isothiocyanate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H314:Skin corrosion/irritation H317:Sensitisation, Skin H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Methyl isothiocyanate
| InChIKey | LGDSHSYDSCRFAB-UHFFFAOYSA-N |
Methyl isothiocyanate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 556-61-6 Methyl isothiocyanate 98%View Details
556-61-6 Methyl isothiocyanate 98%View Details
556-61-6 -
 556-61-6 98%View Details
556-61-6 98%View Details
556-61-6 -
 Methyl isothiocyanate 556-61-6 98%View Details
Methyl isothiocyanate 556-61-6 98%View Details
556-61-6 -
 Methyl isothiocyanate CAS 556-61-6View Details
Methyl isothiocyanate CAS 556-61-6View Details
556-61-6 -
 TRKA (440-end), active, GST tagged human CAS 152787-58-1View Details
TRKA (440-end), active, GST tagged human CAS 152787-58-1View Details
152787-58-1 -
 ANTI-MT1A antibody produced in mouse CASView Details
ANTI-MT1A antibody produced in mouse CASView Details -
 ANTI-TRKA (CENTER) antibody produced in rabbit CASView Details
ANTI-TRKA (CENTER) antibody produced in rabbit CASView Details -
 ANTI-PHOSPHO-TRKA(Y791) antibody produced in rabbit CASView Details
ANTI-PHOSPHO-TRKA(Y791) antibody produced in rabbit CASView Details
