CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | Pale yellow powder |
| Melting Point | 222-223 °C |
| Solubility | Soluble |
| LogP | 3.2 |
| Optical Rotation | Slightly pale yellow needles or crystalline powder from water, mp 256.6 °C. MW 677.18. Specific optical rotation: +67.7 deg at 20 °C/D ( c = 1 in water). UV max (ethanol): 221, 254, 359, 372 nm (epsilon 53800, 36600, 26200, 25300). Slightly soluble in water and organic solvents. pH of aqueous solution = 4. /Hydrochloride trihydrate/ |
| Dissociation Constants | 10.9 |
| Other Experimental Properties | Pale yellow to yellow crystalline powder. MW: 677.19. Slightly soluble in water and organic solvents /Hydrochloride/ |
COMPUTED DESCRIPTORS
| Molecular Weight | 586.7 g/mol |
|---|---|
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 586.27913494 g/mol |
| Monoisotopic Mass | 586.27913494 g/mol |
| Topological Polar Surface Area | 113 Ų |
| Heavy Atom Count | 43 |
| Formal Charge | 0 |
| Complexity | 1200 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
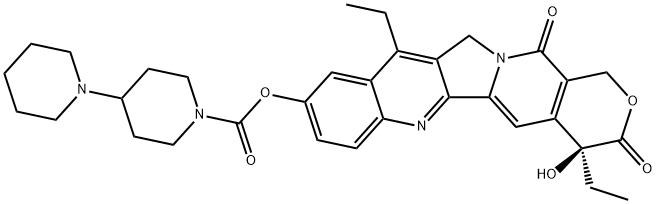
Irinotecan is a member of the class of pyranoindolizinoquinolines that is the carbamate ester obtained by formal condensation of the carboxy group of [1,4'-bipiperidine]-1'-carboxylic acid with the phenolic hydroxy group of (4S)-4,11-diethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2- hydrochloride]quinoline-3,14-dione. Used (in the form of its hydrochloride salt trihydrate) in combination with fluorouracil and leucovorin, for the treatment of patients with metastatic adenocarcinoma of the pancreas after disease progression following gemcitabine-based therapy. It is converted via hydrolysis of the carbamate linkage to its active metabolite, SN-38, which is ~1000 times more active. It has a role as an apoptosis inducer, an EC 5.99.1.2 (DNA topoisomerase) inhibitor, an antineoplastic agent and a prodrug. It is a pyranoindolizinoquinoline, a N-acylpiperidine, a carbamate ester, a tertiary alcohol, a tertiary amino compound, a delta-lactone and a ring assembly. It is functionally related to a SN-38. It is a conjugate base of an irinotecan(1+).