Iprobenfos
- CAS NO.:26087-47-8
- Empirical Formula: C13H21O3PS
- Molecular Weight: 288.34
- MDL number: MFCD00191226
- EINECS: 247-449-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-04-23 13:52:06
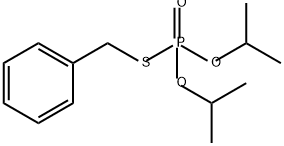
What is Iprobenfos?
The Uses of Iprobenfos
Iprobenfos is a systemic fungicide which provides effective protective and curative control of leaf and ear blast (Pyricularia oryzae), stem rot (Helminthosporium spp.) and sheath blight (Rhizoctonia solani) in rice.
Definition
ChEBI: An organic thiophosphate that is the S-benzyl O,O-diisopropyl ester of phosphorothioic acid. Used as a rice fungicide to control leaf and ear blast, stem rot and sheath blight.
Metabolic pathway
Iprobenfos undergoes extensive degradation and metabolism. Primary metabolic pathways include isomerisation to the thionate (P=S) ester, cleavage of the P-S-benzyl and P-O-isopropyl moieties, and transesterification. Benzyl mercaptan, upon cleavage, undergoes additional oxidation reactions to yield the alcohol, carboxylic acid, disulfide and sulfonic acid. The formation of the oxon from the isomerisation product (P=S ester) was minor and was observed only under UV light irradiation. The primary metabolic pathways of iprobenfos are presented in Scheme 1.
Degradation
Iprobenfos (1) is stable to hydrolytic degradation (<50 °C). The DTN
value of iprobenfos in aqueous solution was approximately 301-324 days(PM).
Iprobenfos degraded rapidly when deposited as a thin film and
exposed to UV light (DT50 ca. 10 min). Two major degradation reactions
were observed during the initial phase of the irradiation. The first reaction
involved the isomerisation of iprobenfos to O,O-diisopropyl O-
benzyl phosphorothioate (2) followed by the oxidative desulfuration
of compound 2 to yield the corresponding oxon analogue 3 (O,O-diisopropyl
O-benzyl phosphate). The second reaction involved the
hydrolytic cleavage of the S-C linkage of iprobenfos to yield O,O-diisopropyl
hydrogen phosphorothioate (4) and the cleavage of the
P-O-benzyl linkage of compound 3 to yield O,O-diisopropyl hydrogen
phosphate (5). Other major photolytic degradation reactions include the
cleavage of the parent P-S linkage to O,O-diisopropyl phosphonate (6).
Benzyl alcohol (7) and benzyl mercaptan (8) were also detected. Further
oxidation of compound 7 yielded benzoic acid (9) and the oxidation
of compound 8 yielded benzylsulfonic acid (10), dibenzyl disulfide (11)
and sulfuric acid as terminal products. Other minor products detected
included transesterification products [O,O,S-triisopropyl phosphorothioate
(12), O,O,O-triisopropyl phosphate (13)] and benzyl isopropyl
sulfide (14).
Properties of Iprobenfos
| Melting point: | 25°C |
| Boiling point: | 126°C |
| Density | 1.1001 g/cm3 |
| vapor pressure | 2.5 x 10-4 Pa (20 °C) |
| refractive index | approximate 1.51 |
| Flash point: | 11 °C |
| storage temp. | APPROX 4°C
|
| form | liquid |
| Water Solubility | 430 mg l-1(20 °C) |
| BRN | 1974687 |
| CAS DataBase Reference | 26087-47-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Kitazin p(26087-47-8) |
| EPA Substance Registry System | Phosphorothioic acid, O,O-bis(1-methylethyl) S-(phenylmethyl) ester (26087-47-8) |
Safety information for Iprobenfos
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Iprobenfos
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 Iprobenfos CAS 26087-47-8View Details
Iprobenfos CAS 26087-47-8View Details
26087-47-8 -
 Iprobenfos CAS 26087-47-8View Details
Iprobenfos CAS 26087-47-8View Details
26087-47-8 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4