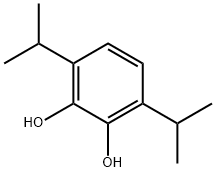
Propofol
Synonym(s):2,6-Bis(1-methylethyl)phenol;2,6-Bis(isopropyl)phenol;2,6-Diisopropylphenol;Propofol
- CAS NO.:2078-54-8
- Empirical Formula: C12H18O
- Molecular Weight: 178.27
- MDL number: MFCD00008885
- EINECS: 218-206-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
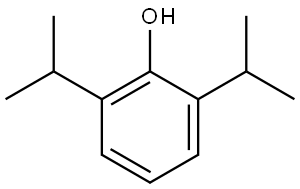
What is Propofol?
Absorption
Rapid - time to onset of unconsciousness is 15-30 seconds, due to rapid distribution from plasma to the CNS. Distribution is so rapid that peak plasma concentrations cannot be readily measured. Duration of action is 5-10 minutes.
Toxicity
Overdosage may increase pharmacologic and adverse effects or cause death.
IV LD50=53 mg/kg (mice), 42 mg/kg (rats). Oral LD50 (as a solution in soybean oil)=1230 mg/kg (mice), 600 mg/kg (rats)
Description
Propofol is an injectable, short-acting general anesthetic with a low incidence of side effects.
Chemical properties
Light Yellow Liquid
Originator
ICI (United Kingdom)
The Uses of Propofol
Propofol is an anesthetic used in veterinary medicine.
The Uses of Propofol
A anesthetic used in veterinary medicine
The Uses of Propofol
2,6-DIPP is the active ingredient for intravenous anesthetic formulations, also has applications as an intermediate for polymers
Background
Propofol is an intravenous anaesthetic agent used for induction and maintenance of general anaesthesia. IV administration of propfol is used to induce unconsciousness after which anaesthesia may be maintained using a combination of medications. Recovery from propofol-induced anaesthesia is generally rapid and associated with less frequent side effects (e.g. drowsiness, nausea, vomiting) than with thiopental, methohexital, and etomidate. Propofol may be used prior to diagnostic procedures requiring anaesthesia, in the management of refractory status epilepticus, and for induction and/or maintenance of anaesthesia prior to and during surgeries.
Indications
Propofol is used for induction and/or maintenance of anaesthesia and for management of refractory status epilepticus.
Definition
ChEBI: Propofol is a phenol resulting from the formal substitution of the hydrogen at the 2 position of 1,3-diisopropylbenzene by a hydroxy group. It has a role as an intravenous anaesthetic, a sedative, a radical scavenger, an antiemetic and an anticonvulsant.
brand name
Diprivan (Abraxis);Disoprivan;Disprofol;Rapinovet.
World Health Organization (WHO)
Propofol, a short acting injectable anaesthetic, was introduced in 1987. In April 1992, the Norwegian Medicines Control Board reported that prolonged use of propofol had been associated with two fatalities in children characterized by metabolic acidosis, liver enlargement, and cerebral oedema. The UK Committee on the Safety of Medicines has received 5 reports of deaths occurring in children who had received propofol while in intensive care.
Biological Functions
Propofol (Diprivan) is rapidly acting, has a short recovery
time, and possesses antiemetic properties. A rapid
onset of anesthesia (50 seconds) is achieved, and if no
other drug is administered, recovery will take place in 4
to 8 minutes.The recovery is attributed to redistribution
of the drug and rapid metabolism to glucuronide and
sulfate conjugates by the liver and extrahepatic tissues,
such as intestine and kidney.
Rapid recovery and its antiemetic properties make
propofol anesthesia very popular as an induction agent
for outpatient anesthesia. Propofol can also be used to
supplement inhalational anesthesia in longer procedures.
Both continuous infusion of propofol for conscious
sedation and with opioids for the maintenance of
anesthesia for cardiac surgery are acceptable techniques.
Synthesis Reference(s)
The Journal of Organic Chemistry, 21, p. 712, 1956 DOI: 10.1021/jo01112a621
General Description
Propofol is an injectable sedative–hypnotic used for the inductionand maintenance of anesthesia or sedation. Propofolis only slightly soluble in water with an octanol/water partitioncoefficient of 6,761:1; thus, it is formulated as an oil-inwateremulsion. The fat component of the emulsion consistsof soybean oil, glycerol, and egg lecithin. The pKa of thepropanol hydroxyl is 11 and the injectable emulsion has apH of 7 to 8.5. Formulations contain either disodium ethylenediaminetetraaceticacid (EDTA) (0.005%) or sodiummetabisulfite to retard the growth of microorganisms. EDTAis a metal chelator and patients on propofol containingEDTA for extended periods of time excrete more zinc andiron in their urine. The clinical consequence of this is notknown but the manufacturer recommends that a drug holidayor zinc supplementation be considered after 5 days of therapy.
Biological Activity
Intravenous general anesthetic and hypnotic with a mode of action which includes potentiation of GABA-mediated inhibitory synaptic transmission, direct activation of the GABA A receptor and inhibition of glutamate receptor mediated excitatory synaptic transmission. Also potentiates P2X 4 receptor-mediated currents in P2X 4 -HEK293 cells.
Pharmacokinetics
Propofol is a sedative-hypnotic agent for use in the induction and maintenance of anesthesia or sedation. Intravenous injection of a therapeutic dose of propofol produces hypnosis rapidly with minimal excitation, usually within 40 seconds from the start of an injection (the time for one arm-brain circulation).
Pharmacology
Propofol is primarily a hypnotic drug with substantial cardiorespiratory depressant actions and with no ability to produce neuromuscular blockade. While propofol lacks analgesic properties, its use permits lower doses of opioids. Likewise, less propofol is required for adequate hypnosis when it is administered with opioids.Thus, it is said that propofol and opioids interact synergistically.
Clinical Use
Generic formulations of propofol may contain sodiummetabisulfite as the antimicrobial agent, and patients allergicto sulfites, especially asthmatic patients, should avoid thisformulation. Aseptic technique must be followed and unusedportions of the drug must be discarded according to the manufacturer’s instructions to prevent microbial contaminationand possible sepsis.
Side Effects
The dose of propofol should be reduced in older patients;
however, it does have a relatively linear dose–
response characteristic, and patients generally can be safely titrated. The pain on injection, especially when
small veins are used, can be considerably reduced if lidocaine
20 mg is administered first.
Anesthesia induction with propofol causes a significant
reduction in blood pressure that is proportional to
the severity of cardiovascular disease or the volume status
of the patient, or both. However, even in healthy patients
a significant reduction in systolic and mean arterial
blood pressure occurs. The reduction in pressure
appears to be associated with vasodilation and myocardial
depression. Although propofol decreases systemic
vascular resistance, reflex tachycardia is not observed.
This is in contrast to the actions of thiopental.
The heart rate stabilization produced by propofol relative
to other agents is likely the result of either resetting
or inhibiting the baroreflex, thus reducing the tachycardic
response to hypotension.
Since propofol does not depress the hemodynamic
response to laryngoscopy and intubation, its use may
permit wide swings in blood pressure at the time of induction
of anesthesia. Propofol should be used with utmost
caution in patients with cardiac disease.
Safety Profile
Poison by intravenous and intraperitoneal routes. Experimental reproductive effects. Combustible when exposed to heat or flame; can react with oxidizing materials. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and fumes. See also PHENOL
Veterinary Drugs and Treatments
In appropriate patients, propofol may be useful as an induction
agent (especially before endotracheal intubation or an inhalant anesthetic),
and as an anesthetic for outpatient diagnostic or minor
procedures (e.g., laceration repair, radiologic procedures, minor
dentistry, minor biopsies, endoscopy, etc.).
Propofol is used as a treatment for refractory status epilepticus,
as it tends to cause less cardiovascular depression and recoveries can
be smoother than with pentobarbital. Propofol may be of particular
usefulness for use in Greyhounds and in patients with preexisting
cardiac dysrhythmias. At low dosages, propofol is being investigated
as an appetite stimulant in dogs.
Propofol may be safely used in animals with liver or renal disease
and mild to moderate cardiac disease.
In dogs, propofol’s labeled indications are: 1) for induction of
anesthesia; 2) for maintenance of anesthesia for up to 20 minutes;
3) for induction of general anesthesia where maintenance is provided
by inhalant anesthetics.
Effects
Propofol is a fast-acting, short-lived intravenous anesthetic. It is particularly effective for outpatient surgery because of rapid postsurgery recovery. Known for its anti-emetic effect, it is currently being tested for oral bioavailability for use with cancer patients.
Metabolism
Hepatically metabolized mainly by glucuronidation at the C1-hydroxyl. Hydroxylation of the benzene ring to 4-hydroxypropofol may also occur via CYP2B6 and 2C9 with subsequent conjugation to sulfuric and/or glucuronic acid. Hydroxypropofol has approximately 1/3 of hypnotic activity of propofol.
Properties of Propofol
| Melting point: | 18 °C (lit.) |
| Boiling point: | 256 °C/764 mmHg (lit.) |
| Density | 0.962 g/mL at 25 °C (lit.) |
| vapor pressure | 5.6 mm Hg ( 100 °C) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | Very slightly soluble in water, miscible with hexane and with methanol |
| form | neat |
| pka | pKa 11.10(H2O,t =20)(Approximate) |
| form | <18°C Solid,>18°C Liquid |
| color | Pale Yellow to Yellow |
| Odor | at 100.00?%. mild phenolic |
| Water Solubility | Very slightly soluble in water. |
| Merck | 14,7834 |
| BRN | 1866484 |
| CAS DataBase Reference | 2078-54-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Phenol, 2,6-bis(1-methylethyl)-(2078-54-8) |
| EPA Substance Registry System | 2,6-Diisopropylphenol (2078-54-8) |
Safety information for Propofol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Propofol
| InChIKey | OLBCVFGFOZPWHH-UHFFFAOYSA-N |
Propofol manufacturer
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran
You may like
-
 Propofol 98%View Details
Propofol 98%View Details -
 2078-54-8 Propofol 99%View Details
2078-54-8 Propofol 99%View Details
2078-54-8 -
 Propofol CAS 2078-54-8View Details
Propofol CAS 2078-54-8View Details
2078-54-8 -
 2078-54-8 98%View Details
2078-54-8 98%View Details
2078-54-8 -
 2,6-Diisopropylphenol CAS 2078-54-8View Details
2,6-Diisopropylphenol CAS 2078-54-8View Details
2078-54-8 -
 Propofol 2078-54-8 98%View Details
Propofol 2078-54-8 98%View Details
2078-54-8 -
 Propofol CAS 2078-54-8View Details
Propofol CAS 2078-54-8View Details
2078-54-8 -
 Propofol solution CAS 2078-54-8View Details
Propofol solution CAS 2078-54-8View Details
2078-54-8
