IOXILAN (400 MG)
- CAS NO.:107793-72-6
- Empirical Formula: C18H24I3N3O8
- Molecular Weight: 791.11
- MDL number: MFCD00867280
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
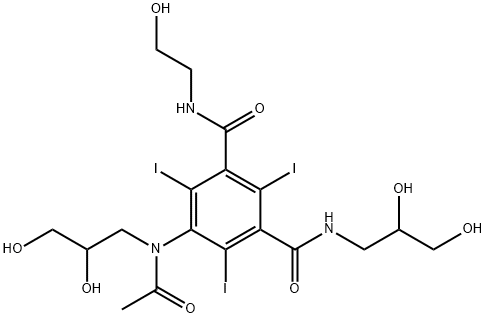
What is IOXILAN (400 MG)?
Absorption
Peak iodine plasma levels occur immediately following rapid intravenous injection. Iodine plasma levels fall rapidly within 5 to 10 minutes. This can be accounted for by the dilution in the vascular and extravascular fluid compartments.
Toxicity
Renal toxicity has been reported in a few patients with liver dysfunction who were given an oral cholecystographic agent followed by intravascular contrast agents. Administration of any intravascular contrast agent should therefore be postponed in any patient with a known or suspected hepatic or biliary disorder who has recently received a cholecystographic contrast agent.
Other drugs should not be admixed with this product.
Pregnancy Category B
The Uses of IOXILAN (400 MG)
Nonionic iodinated contrast medium. Diagnostic aid (radiopaque medium).
Background
Ioxilan is a tri-iodinated diagnostic contrast agent. Intravascular injection results in opacification of vessels in the path of flow of the contrast medium, permitting radiographic visualization of the internal structures of the human body until significant hemodilution occurs.
Indications
When administered intra-arterially, Ioxilan is indicated for the following diagnostic tests: cerebral arteriography (300 mgI/mL), coronary arteriography and left ventriculography (350 mgI/mL), visceral angiography(350 mgI/mL), aortography(350 mgI/mL), and peripheral arteriography(350 mgI/mL). When administered intravenously, Ioxilan is indicated for excretory urography and contrast enhanced computed tomographic (CECT) imaging of the head and body (300 and 350 mgI/mL).
Definition
ChEBI: Ioxilan is an amidobenzoic acid.
brand name
Oxilan (Guerbet).
Pharmacokinetics
As with other iodinated contrast agents the degree of contrast enhancement is directly related to the iodine content in the administered dose.
Metabolism
There is no evidence for metabolism.
Properties of IOXILAN (400 MG)
| Boiling point: | 827.8±65.0 °C(Predicted) |
| Density | 2.205±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO: ≥ 250 mg/mL (316.01 mM) |
| pka | 11.47±0.46(Predicted) |
| form | Solid |
| color | White to off-white |
| EPA Substance Registry System | 1,3-Benzenedicarboxamide, 5-[acetyl(2,3-dihydroxypropyl)amino]-N1-(2,3-dihydroxypropyl)-N3-(2-hydroxyethyl)-2,4,6-triiodo- (107793-72-6) |
Safety information for IOXILAN (400 MG)
Computed Descriptors for IOXILAN (400 MG)
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1