Iopamidol
- CAS NO.:60166-93-0
- Empirical Formula: C17H22I3N3O8
- Molecular Weight: 777.09
- MDL number: MFCD00867931
- EINECS: 262-093-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 11:34:44
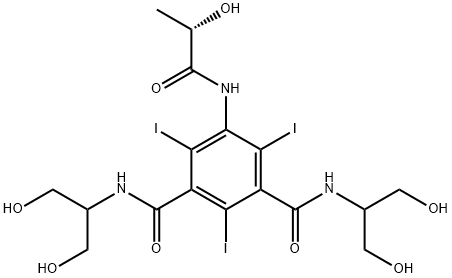
What is Iopamidol?
Chemical properties
White Crystalline Powder
Originator
Iopamiro,Bracco,Italy,1981
The Uses of Iopamidol
Iopamidol is an organic iodine compound and used as a nonionic radiocontrast medium. Diagnostic aid (radiopaque medium). Iopamidol blocks x-rays as they pass through the body, thereby allowing body structures not containing iodine to be visualized. The degree of opacity produced by iopamidol is directly proportional to the total amount of the iodinated contrast agent in the path of the x-rays. The visualization of body structures is dependent upon the distribution and elimination of iopamidol. (NCI05)
Background
Iopamidol is a contrast agent developed by Bracco with nonionic, low-osmolar properties.
What are the applications of Application
Iopamidol is a nonionic radiocontrast medium
Definition
ChEBI: Iopamidol is a benzenedicarboxamide compound having N-substituted carbamoyl groups at the 1- and 3-positions, iodo substituents at the 2-, 4- and 6-positions and a (2S)-2-hydroxypropanamido group at the 5-position. It has a role as a radioopaque medium, an environmental contaminant and a xenobiotic. It is a benzenedicarboxamide, an organoiodine compound and a pentol.
Manufacturing Process
400 g (0.72 mol) 5-amino-2,4,6-triiodo-isophthalic acid was added to 200 ml
thionyl chloride, the mixture was stirred at a boil for 6 hours, and the
resulting solution was evaporated. The residue was dissolved in anhydrous
ethyl acetate, and the solution was again evaporated to dryness. The solid
material was dissolved in 4,000 ml ethyl acetate, and the solution was stirred
into an ice-cold solution of 500 g sodium chloride and 200 g sodium
bicarbonate in 2.5 liters water. The organic phase was separated from the
aqueous solution, washed with aqueous sodium solution, dried by contact with
anhydrous calcium chloride, and evaporated to dryness.
The residue of 420 g 5-amino-2,4,6-triiodo-isophthalyl chloride (97.5% yield)
had a melting point above 300°C when recrystallized from toluene.
300 g (0.503 mol) 5-amino-2,4,6-triiodo-isophthalyl chloride was dissolved in
1,200 ml dimethylacetamide, and 187 g (126 mol) DL-2-acetoxypropionyl
chloride was added dropwise to the solution with agitation. The mixture was
permitted to stand overnight at ambient temperature and was then
evaporated in a vacuum to approximately 400 ml. The oily residue was stirred
into ice water to precipitate 353 g crystalline DL-5-(α-acetoxypropionylamino)-
2,4,6-triiodo-isophthalyl chloride (98% yield) which was purified by
suspension in warm chloroform free alcohol.
The purified intermediate melted at 210°C. 70.9 g (0.10 mol) of the
intermediate was dissolved in 150 ml dimethylacetamide, and 15 g (0.08 mol)
tributylamine was added. The mixture was heated to 50°C, and 56.6 g (0.62
mol) 1,3-dihydroxyisopropylamine (2-amino-1,3-propanediol) dissolved in 80
ml dimethylacetamide was added drop by drop. The reaction went to
completion within a few hours, and the reaction mixture was evaporated to
dryness in a vacuum. The oily residue was added to 350 ml methylene
chloride with vigorous agitation, and the resulting precipitate was filtered off
and purified by repeated suspension of warm methylene chloride.
Work-up of the reaction mixture yielded 56.5 g (73.5%) DL-5-α-
hydroxypropionylamino-2,4,6-triiodo-isophthalic acid di-(1,3-
dihydroxyisopropylamide) which was recrystallized from aqueous ethanol and
melted with decomposition above 300°C.
brand name
Isovue (Bracco).
Therapeutic Function
Diagnostic aid (radiopaque medium)
General Description
Iopamidol is a low-osmolar, nonionicmonomer with 49% organically bound iodine. It is indicatedfor use in angiography, excretory urography, andnumerous CT procedures.
Side Effects
Arm, back, or jaw pain; blurred vision; chest pain or discomfort; chest tightness or heaviness; confusion; dizziness, faintness, or lightheadedness when getting up suddenly from a lying or sitting position; fast or irregular heartbeat; feeling of warmth; hives; lightheadedness, dizziness, or fainting; nausea; redness of the face, neck, arms, and occasionally, upper chest; slow or irregular heartbeat; sudden sweating; trouble breathing; unusual tiredness or weakness
Toxicology
Serious skin reactions , including Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis can occur with this medicine. Results from in vitro HEK293T cell-based assays indicate that iopamidol affects mitochondrial function Treatment with iopamidol induces ATP depletion, reduces the mitochondrial membrane potential, and elevates mitochondrial superoxide and reactive oxygen species accumulation.
Metabolism
Not Available
Mode of action
Iopamidol is a Radiographic Contrast Agent. The mechanism of action of iopamidol is as a X-Ray Contrast Activity.
Properties of Iopamidol
| Melting point: | >3200C (dec) |
| alpha | D20 -2.01° (c = 10 in water) |
| Boiling point: | 740.14°C (rough estimate) |
| Density | 2.0203 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | Freely soluble in water, very slightly soluble in methanol, practically insoluble in ethanol (96 per cent) and in methylene chloride |
| form | neat |
| pka | pKa (25°) 10.70 |
| form | Solid |
| color | White to off-white |
| Water Solubility | 473.7g/L(25 ºC) |
| CAS DataBase Reference | 60166-93-0(CAS DataBase Reference) |
Safety information for Iopamidol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Iopamidol
Iopamidol manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Iopamidol 98%View Details
Iopamidol 98%View Details -
 60166-93-0 99%View Details
60166-93-0 99%View Details
60166-93-0 -
 Iopamidol 99%View Details
Iopamidol 99%View Details
60166-93-0 -
 Iopamidol 60166-93-0 98%View Details
Iopamidol 60166-93-0 98%View Details
60166-93-0 -
 Iopamidol 99%View Details
Iopamidol 99%View Details
60166-93-0 -
 Iopamidol >98%View Details
Iopamidol >98%View Details -
 IPAMIDOL 60166-93-0 95-99%View Details
IPAMIDOL 60166-93-0 95-99%View Details
60166-93-0 -
 Iopamidol CAS 60166-93-0View Details
Iopamidol CAS 60166-93-0View Details
60166-93-0
