IONOMYCIN
Synonym(s):Ionomycin, Free Acid, Streptomyces conglobatus - CAS 56092-81-0 - Calbiochem;Ionomycin, Free Acid, Streptomyces conglobatus in Solution - CAS 56092-81-0 - Calbiochem;SQ23377 calcium
- CAS NO.:56092-81-0
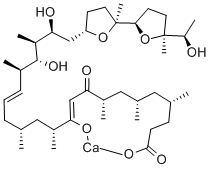
- Empirical Formula: C41H72O9
- Molecular Weight: 709.01
- MDL number: MFCD06798385
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-18 21:14:07
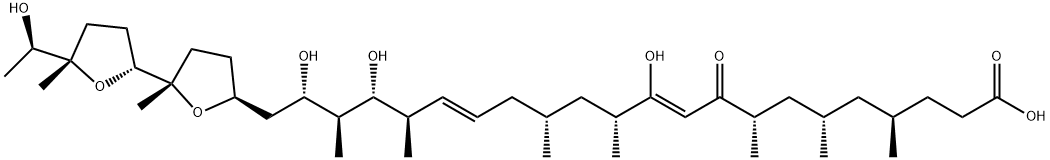
What is IONOMYCIN?
Description
Ionomycin (56092-81-0) is a highly selective nonfluorescent calcium (Ca2+) ionophore.1 Induces a rapid rise in cytosolic Ca2+ in human neutrophils which is due to both release from cytosolic Ca2+ stores as well as Ca2+ influx.2 It activates (2 μM) and primes (20-200 nM) neutrophil NADPH oxidase2. Down regulates beta-catenin/Tcf signaling in a colon cancer cell line via suppressing the binding of Tcf to its specific DNA-binding site.3 In rat hepatoma cells, sub-lethal ionomycin activates the stress response by activating SAPK/JNK and HSF/HSE interaction leading to upregulation of HSP70 biosynthesis.4
Chemical properties
White powder
The Uses of IONOMYCIN
Ionomycin was isolated from Streptomyces conglobatus as a potent Gram positive antibiotic . During isolation, it was recognised that ionomycin exhibits a very high affinity and selectivity for calcium ions, suggesting the metabolite acts as a calcium ionophore. More recently, ionomycin has been used in cell biology as a universal calcium ionophore to explore the role of calcium regulation in the cell.
The Uses of IONOMYCIN
Ionomycin is more effective than A23187 as a Ca++ionophore. Ionomycin is used in research on Ca++ transport across biological membranes; Ionomycin induces apoptotic degeneration of embryonic cortical neurons
The Uses of IONOMYCIN
Ionomycin free acid is a beneficial tool in research to raise intracellular calcium levels. Also this antibiotic can be used to study the calcium transport across the plasma membrane. It has been shown to induce central demyelination , inhibit adrenal bovine TREK-1 channels , and to regulate cell division of mature human B cells . It is used to study the effects of calcium flux on endoplasmic reticulum (ER) stress, mitochondrial stress and intrinsic apoptosis mechanisms. It is also used to stimulate the intracellular production of the cytokines, interferon, perforin, IL-2, and IL-4 usually in conjunction with PMA.
What are the applications of Application
Ionomycin free acid is a potent and selective calcium ionophore antibiotic, able to extract calcium ions from aqueous phase to organic phase.
Definition
ChEBI: A very long-chain fatty acid that is docosa-10,16-dienoic acid which is substituted by methyl groups at positions 4, 6, 8, 12, 14, 18 and 20, by hydroxy groups at positions 11, 19 and 21, and by a (2',5-dimethyloctahydro-2,2'-bifuran-5-yl)ethanol group at osition 21. An ionophore produced by Streptomyces conglobatus, it is used in research to raise the intracellular level of Ca2+ and as a research tool to understand Ca2+ transport across biological memb anes.
General Description
Highly specific for divalent cations (Ca2+ > Mg2+ >> Sr2+ = Ba2+). More effective than antibiotic A23187 as a mobile ion carrier for Ca2+. Complexes with Ca2+ between pH 7 and 9.5, causing significant absorption in the UV range; however, in contrast to A23187 (Cat. No. 100105), it is not fluorescent. Useful for studies of Ca2+ transport across biological membranes and measurement of cytoplasmic free Ca2+. Induces apoptotic neuronal degeneration in embryonic cortical neurons. Also induces cell cycle arrest in the G1 interval of mature Burkitt lymphoma cell lines.
Biological Activity
Calcium ionophore; more specific than A23187 (5-(Methylamino)-2-[[2R,3R,6S,8S,9R,11R)-3,9,11-trimethyl-8-[(1S)-1-methyl-2-oxo-2-(1H-pyrrol-2-yl)-ethyl]-1,7-dioxaspiro[5.5]undec-2-yl]methyl]-4-benzoxazolecarboxylic acid ).
Biochem/physiol Actions
Cell permeable: yes
in vitro
calcium ion can be extracted by ionomycin fom the queous phase into the organic phase. ionomycin also acts as a mobile ion carrier, which transports the cation across a solvent barrier [1].
in vivo
efficiency of ionomycin on oocyte activation and subsequent development was evaluated, which identified ionomycin as an efficient activator at the concentation of 10 μmol/l. with a second exposure to 5 μmol/l ionomycin on blastocyst development, an improved effect was found. no adverse effects of ionomycin on mouse embryo development were identified [2].
storage
-20°C
References
1) Kaufmann et al., (1980), Cation transport and specificity of ionomycin. Comparison with ionophore A23187 in rat liver mitochondria; J. Biol. Chem. 255 2735 2) Elzi et al., (2001), Ionomycin causes activation of p38 and p42/44 mitogen-activated protein kinases in human neutrophils; Am. J. Physiol. Cell Physiol. 281 C350 3) Park et al., (2005), Ionomycin downregulates beta-catenin/tcf signaling in colon cancer cell line; Carcinogenesis, 26 1929 4) Sreedhar and Srinivas (2002), Activation of stress response by ionomycin in rat hepatoma cells; J. Cell Biochem., 86 154
Properties of IONOMYCIN
| Boiling point: | 817.2±65.0 °C(Predicted) |
| Density | 1.072±0.06 g/cm3(Predicted) |
| storage temp. | −20°C |
| solubility | Soluble to 10 mM in Ethanol and to 10 mM in DMSO. |
| form | Waxy solid |
| pka | 4.77±0.10(Predicted) |
| color | Yellow |
| BRN | 3642126 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
Safety information for IONOMYCIN
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H225:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for IONOMYCIN
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Ionomycin from Streptomyces conglobatus CAS 56092-81-0View Details
Ionomycin from Streptomyces conglobatus CAS 56092-81-0View Details
56092-81-0 -
 Ionomycin, Free Acid, Streptomyces conglobatus CAS 56092-81-0View Details
Ionomycin, Free Acid, Streptomyces conglobatus CAS 56092-81-0View Details
56092-81-0 -
 Ionomycin, Free Acid, Streptomyces conglobatus in Solution CAS 56092-81-0View Details
Ionomycin, Free Acid, Streptomyces conglobatus in Solution CAS 56092-81-0View Details
56092-81-0 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0