iodoxamic acid
- CAS NO.:31127-82-9
- Empirical Formula: C26H26I6N2O10
- Molecular Weight: 1287.92
- MDL number: MFCD00867934
- EINECS: 250-478-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:37
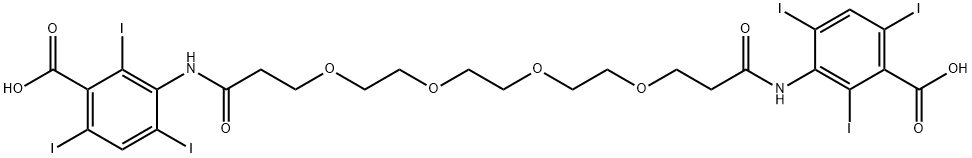
What is iodoxamic acid?
Originator
Endobil,Bracco
The Uses of iodoxamic acid
Diagnostic aid (radiopaque medium).
Definition
ChEBI: Iodoxamic acid is an organic molecular entity.
Manufacturing Process
148.5 g 4,7,10,13-tetraoxahexadecane-1,16-dinitrile (U.S. Patent No.
2,401,607) was added to a solution of 232 g (2.45 mol) concentrate sulfuric
acid in 290 ml absolute ethanol at 15°C. The mixture was heated at reflux for
15 hours, cooled and poured into 1000 g ice and 250 g ammonium sulfate. It
was extracted with methylene chloride, dried and a solvent was removed in
vacuum. The residue was distilled to give 4,7,10,13-tetraoxahexadecane-1,16-
dicarbonic acid dimethyl ester; BP: 190°-195°C/0.005 mm Hg.
1 mol above prepared diester was saponificated with equivalent of NaOH in
water. The reaction mixture was heated for 90 minutes. On cooling it was
extracted with ether and the water layer was evaporated to dryness. The
residue was washed with acetone. The obtained disodium salt of 4,7,10,13-
tetraoxahexadecane-1,16-dicarbonic acid (yield 100%; MP: 102°-104°C) was
acidified with calculated quantity of HCl to give the dicarbonic acid. The
solvent was evaporated to dryness. Acetone was added to the residue for
removing a by-product (sodium chloride) by filtration. Acetone was evaporated
and the residue was extracted with ether, dried and evaporated. The residual
liquid was 4,7,10,13-tetraoxahexadecane-1,16-dicarbonic acid.
100 ml thionyl chloride was cautious added to 56 g above prepared diacid and
heated at 40°-50°C and excess thionyl chloride was distilled in vacuum. The
residue was the 4,7,10,13-tetraoxahexadecane-1,16-dicarbonic acid
dichloride. The desired iodoxamic acid was prepared from above dichloride
and 3-amino-2,4,6-trijode benzoic acid, in dimethylacetamide
brand name
Endobil (Bracco Industria Chimica S.p.A.,Italy); Endomirabil (Bracco Industria Chimica S.p.A., Italy); Videocolangio (Bracco Industria Chimica S.p.A., Italy).
Therapeutic Function
Diagnostic aid
Properties of iodoxamic acid
| Boiling point: | 977.7±65.0 °C(Predicted) |
| Density | 2.423±0.06 g/cm3(Predicted) |
| pka | pKa 1.8/2.8(H2O,t = 25) (Uncertain) |
Safety information for iodoxamic acid
Computed Descriptors for iodoxamic acid
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

![2-[2-(3-aminopropoxy)ethoxy]ethanol](https://img.chemicalbook.in/CAS/GIF/112-33-4.gif)

You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
