Iodine monobromide
Synonym(s):Bromine iodide;Bromoiodine;Iodine monobromide;Iodine solution acc. to Hanus
- CAS NO.:7789-33-5
- Empirical Formula: BrI
- Molecular Weight: 206.81
- MDL number: MFCD00011353
- EINECS: 232-159-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
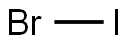
What is Iodine monobromide?
Chemical properties
A dark grey to brownish-black crystalline mass that can be dissolved in ethanol, ether, chloroform, carbon disulphide, and other solvents. When it is heated, it becomes partially dissociated. It can prepared by the action of iodine with bromine in a stream of nitrogen. It is used in organic synthesis.
The Uses of Iodine monobromide
Iodine monobromide is used as an electrophile employed in a new synthetic approach to polyketide structural units. It is also used in iodometry and serves as a source of I+. It is a powerful iodinating agent used in organic synthesis as well as involved in the synthesis of radioiodinated fatty acids for heart imaging.
What are the applications of Application
Iodine monobromide is a synthesis reagent
Preparation
Prepared from the elements, excess Br is removed by heating to 50 °C in a current of CO2.
Hazard
Toxic by ingestion and inhalation, vapors corrosive to tissue.
Health Hazard
Vapors of iodine monobromide are irritatingto the skin, eyes, and mucous membranes.Its concentrated solutions are corrosive to theskin. Toxicity data for this compound are notavailable.
Fire Hazard
Noncombustible solid. The molten salt ex plodes when mixed with potassium, and ignites with finely divided aluminum. Its mixture with sodium explodes on impact. The reaction of phosphorus with molten salt is violent.
Purification Methods
The brown-black crystals are purified by repeated fractional crystallisation from its melt. The vapour dissociates on heating [Yost et al. J Amer Chem Soc 55 552 1933, Schmeisser in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 291-292 1963].
Precautions
corrosive solid and vapor; readily absorbed through skin and mucous membranes; use in a fume hood and wear gloves and appropriate protective clothing; air-, moisture-, and light-sensitive; should be stored refrigerated (under N2) in a tightly sealed amber bottle. Forms explosive mixtures with potassium and sodium, and reacts extremely exothermically with phosphorus and tin.
Properties of Iodine monobromide
| Melting point: | 42-50 °C (lit.) |
| Boiling point: | 116 °C |
| Density | 4.416 g/mL at 25 °C (lit.) |
| Flash point: | 116°C |
| storage temp. | 2-8°C |
| solubility | Aqueous Base (Slightly), Methanol (Slightly), Water (Slightly) |
| form | Solution |
| Specific Gravity | 4.4157 |
| color | Dark red |
| PH | <1 (H2O, 20°C) |
| Water Solubility | Soluble in water, alcohol, ether, carbon disulfide and glacial acetic acid. |
| Sensitive | Moisture & Light Sensitive |
| Merck | 14,5016 |
| BRN | 3902973 |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 7789-33-5(CAS DataBase Reference) |
| EPA Substance Registry System | Iodine bromide (IBr) (7789-33-5) |
Safety information for Iodine monobromide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Iodine monobromide
Iodine monobromide manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


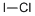
You may like
-
 Iodine monobromide 98%View Details
Iodine monobromide 98%View Details -
 Iodine monobromide CAS 7789-33-5View Details
Iodine monobromide CAS 7789-33-5View Details
7789-33-5 -
 Iodine bromide CAS 7789-33-5View Details
Iodine bromide CAS 7789-33-5View Details
7789-33-5 -
 Iodine monobromide, 98% CAS 7789-33-5View Details
Iodine monobromide, 98% CAS 7789-33-5View Details
7789-33-5 -
 Hanus solution CASView Details
Hanus solution CASView Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
