Ingenol mebutate
- CAS NO.:75567-37-2
- Empirical Formula: C25H34O6
- Molecular Weight: 430.53
- MDL number: MFCD22683801
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-25 20:05:57
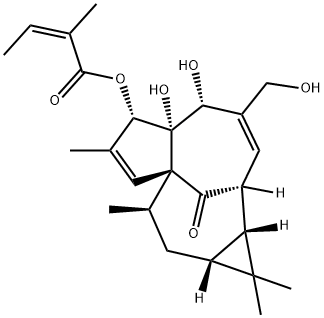
What is Ingenol mebutate?
Absorption
Since ingenol mebutate is a topical treatment, the systemic absorption is less than 0.1 ng/mL.
Toxicity
The most common adverse reactions are local skin reactions at the application site, headache, periorbital edema,and nasopharyngitis.
Description
Ingenol mebutate (also known as PEP005) was approved in January 2012 by the US FDA for the topical treatment of actinic keratoses (AK). Ingenol mebutate also received approval in 2012 in the European Union, Australia, and Brazil for the same indication. Ingenol mebutate, a diterpene ester natural product, is the active agent in the sap of the plant Euphorbia peplus, which has long been used as a traditional remedy for skin lesions. Ingenol mebutate has a novel mechanism of action involving initial plasma membrane disruption, rapid loss of mitochondrial membrane potential, and cell death by primary necrosis within 1 h; a subsequent tumor-specific immune response results in antibodydependent cellular toxicity that eliminates residual cells. Ingenol mebutate is obtained by extraction from the dried, milled aerial parts of Euphorbia peplus followed by a series of purification steps.
Originator
Peplin (United States)
The Uses of Ingenol mebutate
Ingenol 3-Angelate is known to exhibit antitumor activities which induces plasma membrane and mitochondrial disruption and necrotic cell death.
The Uses of Ingenol mebutate
Ingenol 3-Angelate is used as a PKC activator, anticancer, and immunostimulant compound. It is also used to exhibits antileukemic activity, induces nuclear translocation of PKCδ, and induces apoptosis in acute myeloid leukemia (AML) cell lines and primary AML blast cells. Also displays antiproliferative effects and induces apoptosis in Colo205 cells.
Background
Ingenol mebutate was approved by the FDA in January 2012, and it is marketed under the name Picato?. Picato gel is indicated for the topical treatment of actinic keratosis. Before approval, ingenol mebutate was called PEP005 as an investigational drug. PEP005 is a selective small molecule activator of protein kinase C (PKC) extracted from the plant Euphorbia peplus, whose sap has been used as a traditional medicine for the treatment of skin conditions including warts and cancer. PEP005 also has potent anti-leukemic effects, inducing apoptosis in myeloid leukemia cell lines and primary AML cells at nanomolar concentrations.
Indications
For the topical treatment of actinic keratosis.
What are the applications of Application
Ingenol 3-angelate is a PKC activator, anticancer, and immunostimulant compound
Definition
ChEBI: A tetracyclic diterpenoid ester obtained by formal condensation of the carboxy group of (2Z)-2-methylbut-2-enoic (angelic) acid with the 3-hydroxy group of ingenol. Used for the topical treatment of actinic keratosis.
brand name
Picato
Biochem/physiol Actions
Ingenol-3-angelate, is a phorbol ester-like compound or a diacylglycerol analog that is used in the treatment of several disorders like skin cancer. It is also known as PEP005. Ingenol-3-angelate stimulates the initiation of the enzyme by providing PKCs (protein kinase C) to cellular membranes.
Pharmacokinetics
The pharmacodynamics of ingenol mebutate in producing cell death in actinic keratosis is unknown.
Clinical Use
Ingenol mebutate is a diterpene ester which was approved in the US, EU, Austrailia, and Brazil for the treatment of actinic keratosis, a disease stage associated with sun exposure which can potentially develop into cancer. The drug, which is marketed by LEO Pharma A/S as Picato®, is administered as a topical gel (0.015%, 0.05%) which has been proven effective in treating face-, scalp-, and trunklocalized actinic keratosis in four randomized, double-blind, vehicle-controlled, multicenter studies. The drug exhibits mild side effects limited to application-site conditions (e. g. irritation, pain, pruritus), and no detectable concentrations of ingenol mebutate or two of its metabolites were found in blood samples.94 Traditionally used as a home remedy for various skin conditions, the ingenol mebutate, also referred to as ingenol 3-angelate, is the main active constituent of sap from the plant Euphorbia pelpus.95 From natural extractions, 17 kg of fresh E. pelpus afforded 7 g of ingenol 3-angelate as an oil, which upon further purification was deemed insufficient for process-scale production.
Synthesis
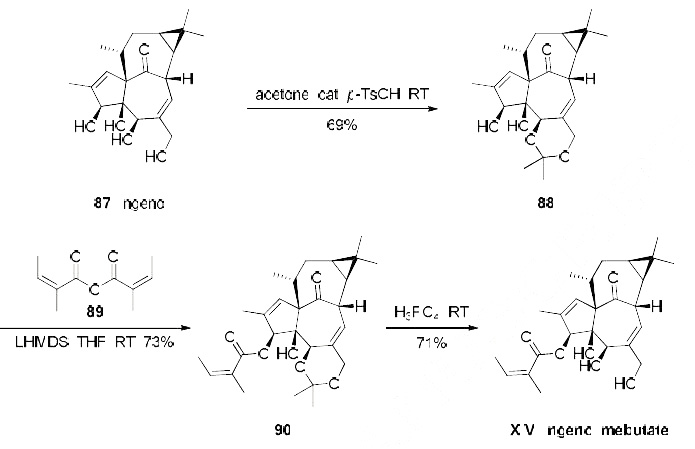
Although several synthetic approaches to the ingenol family of terpenes have been reported,97-113 Liang and coworkers at LEO Pharma have reported a semisynthesis of the API from naturally-occurring ingenol. This natural product?ˉs accessibility from the seeds of E. lathyris renders it widely commercial on scale. The conversion of ingenol to ingenol mebutate involves a protection, esterification, and deprotection strategy to procure scale quantities of the drug.114 Conversion of ingenol (87) to the corresponding 5,20-acetonide 88 proceeded in good yield using a protocol modified from the original conditions described by Hecker. A considerable amount of study was conducted by Liang to affect efficient angeloylation with minimal isomerization of the double-bond to the corresponding Z-isomer (tiglate). It was found that angelic anhydride 89 (which is a commercially available reagent, but for process scale was prepared immediately prior to usage from the self-condensation of 99.5% pure angelic acid with 0.5 equivalents of DCC) in the presence of LHMDS gave acetonide 90 in over 95% conversion and was practically free of the undesired tiglate bi-product after recrystallization (73% yield).96 Deprotection of the acetonide 90 was affected using phosphoric acid and after three recrystallizations, ingenol mebutate (XIV) was produced on multigram scale in a combined yield of 37% starting from ingenol 87.
Metabolism
There is no metabolism of Picato since ingenol mebutate is a topical treatment, and ingenol mebutate does not inhibit or induce a majority of the cytochrome P450 (CYP) enzymes.
storage
-20°C
References
References/Citations
Properties of Ingenol mebutate
| Boiling point: | 576.9±50.0 °C(Predicted) |
| Density | 1.26±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: soluble15mg/mL, clear |
| pka | 11.52±0.70(Predicted) |
| form | powder |
| color | white to beige |
| Water Solubility | Soluble in 100% ethanol, DMSO, dichloromethane, and methanol. Insoluble in water. |
| Stability: | Stable for 1 year as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 1 month. |
Safety information for Ingenol mebutate
Computed Descriptors for Ingenol mebutate
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium 3-Bromopyrazole (3aR,4R,5R,6aS)-hexahydro-5-Triethyl silyloxy-4-((E)-3-oxo-5-phenylpent-1- enyl)cyclopenta[b]furan-2-one. 1-Chlorocarbonyl-4-piperidinopiperidine 1-Bromo-4-phenyl-2-Butanone 4-Amino-2-fluoro-N-methylbenzamide 1,1'-Carbonyldiimidazole SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTIONRelated products of tetrahydrofuran

![4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]-4-piperidyl decanoate](https://img.chemicalbook.in/CAS/GIF/74050-97-8.gif)
![(6R)-6,6aβ,7aβ,8,9,12,12a,12bβ-Octahydro-12α,12aα-dihydroxy-2,2,7,7,9β,11-hexamethyl-7H-6β,9aβ-methano-4H-cyclopenta[9,10]cyclopropa[5,6]cyclodeca[1,2-d]-1,3-dioxin-13-one](https://img.chemicalbook.in/CAS/GIF/77573-43-4.gif)
![(1aR,7bR)-1aα,2,7aα,13,14,14aα-Hexahydro-1,1,6,6,9,9,11,13α-octamethyl-10aαH-2α,12aα-methano-1H,4H-cyclopropa[5,6][1,3]dioxolo[2',3']cyclopenta[1',2':9,10]cyclodeca[1,2-d][1,3]dioxin-15-one](https://img.chemicalbook.in/CAS/GIF/77573-44-5.gif)
You may like
-
 Ingenol-3-angelate CAS 75567-37-2View Details
Ingenol-3-angelate CAS 75567-37-2View Details
75567-37-2 -
![Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
54094-19-8 -
 85-81-4 99%View Details
85-81-4 99%View Details
85-81-4 -
![208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 -
 2033-24-1 99%View Details
2033-24-1 99%View Details
2033-24-1 -
 Meldrums acid 2033-24-1 99%View Details
Meldrums acid 2033-24-1 99%View Details
2033-24-1 -
 Cyaclopentane carboxylic acid 99%View Details
Cyaclopentane carboxylic acid 99%View Details
3400-45-1 -
 2-Aminopyridine 504-29-0 99%View Details
2-Aminopyridine 504-29-0 99%View Details
504-29-0