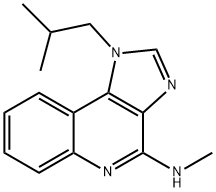
Imiquimod
Synonym(s):1-(2-Methylpropyl)-1H-imidazole[4,5-c]quinoline-4-amine;1-(2-Methylpropyl)-1H-imidazole[4,5-c]quinoline-4-amine;Imiquimod;Imiquimod - CAS 99011-02-6 - Calbiochem
- CAS NO.:99011-02-6
- Empirical Formula: C14H16N4
- Molecular Weight: 240.3
- MDL number: MFCD00866946
- EINECS: 619-387-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-29 14:36:51
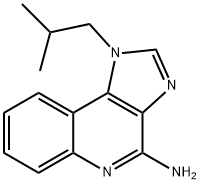
What is Imiquimod?
Absorption
Well absorbed through skin (as a cream)
Toxicity
Symptoms of overdose include flu-like symptoms, such as fever, fatigue, headache, nausea, diarrhoea and muscle pain.
Description
Aldara was launched in the US for the topical treatment of genital warts caused by human papillomavirus (HPV). It can be prepared in a six step approach beginning with the nucleophilic substitution of 4-chloro-3-nitroquinolone with isobutylamine or via a thermal electrocyclic ring closure of 1 - and 2-azahexatriene systems. Aldara has antiviral and antitumor properties, with the former activity arising from the induction of cytokines, in particular, INF-α. Aldara also induces TNF-γ, IL-1α, IL-1β, IL-1ra, IL-6, IL-8, IL-10, GM-CSF, G-CSF and MID-la formation 1-4 hr after stimuli. The exact cells responsible for the response have not been determined, however, it is not activating T lymphocytes, NK cells, B lymphocytes, or dendritic stem cells but monocytes (CD14+, CD36+, HLA-DR+, HLA-DQ±, CD19-, CD16- and CD23-) are partly responsible. The speculation is that Aldara may interact with a kinase modulating the transduction pathway leading to cytokine genes. There is no direct antiviral activity (induction of IFN does not follow true dose response) and it is not mutagenic. The hydroxylated metabolite also induces IFN-α.
Chemical properties
White to off white crystalline powder
Originator
3M Pharmaceuticals (US)
The Uses of Imiquimod
Imiquimod is a caspase 3 activator which acts as an immunomodulator and displays antiviral and anti-tumor activity. It is a patient-applied cream used for the treatment of genital warts and basal cell carcinoma. It is also used to cure actinic keratosis on the face and scalp. It belongs to a group of drugs called immune response modifiers, which work by activating the immune system to fight abnormal skin growth.
The Uses of Imiquimod
An immune response modifier. It stimulates the production of interferon-a
The Uses of Imiquimod
raw material for Latamoxef, Cefminox, Ceftizoxime, Cefoxitin, Cefmetazole
Indications
For the topical treatment of clinically typical, nonhyperkeratotic, nonhypertrophic actinic keratoses on the face or scalp in immunocompetent adults. Also indicated for the treatment of external genital and perianal warts/condyloma acuminata in individuals 12 years old and above.
Background
Imiquimod is an immune response modifier that acts as a toll-like receptor 7 agonist. Imiquimod is commonly used topically to treat warts on the skin of the genital and anal areas. Imiquimod does not cure warts, and new warts may appear during treatment. Imiquimod does not fight the viruses that cause warts directly, however, it does help to relieve and control wart production. Miquimod is also used to treat a skin condition of the face and scalp called actinic keratoses and certain types of skin cancer called superficial basal cell carcinoma.
What are the applications of Application
Imiquimod is a TLR7 activator and angiogenesis inhibitor
Indications
Imiquimod (Aldara) is a topical immune response modifier approved for the treatment of anogenital warts (condylomata acuminata). The exact mechanism of action is unknown; it has no direct antiviral activity in vitro. It is thought to work in vivo by inducing the production of tumor necrosis factor (TNF α ), interferons (IFN)α and γ , and other cytokines with antiviral activity. It may also be useful for treatment of other types of warts, molluscum contagiosum, and certain forms of skin cancer. Local irritant reactions related to the frequency of application are common.
Definition
ChEBI: Imiquimod is an imidazoquinoline fused [4,5-c] carrying isobutyl and amino substituents at N-1 and C-4 respectively. A prescription medication, it acts as an immune response modifier and is used to treat genital warts, superficial basal cell carcinoma, and actinic keratosis. It has a role as an antineoplastic agent and an interferon inducer.
brand name
Aldara (3M Pharmaceuticals).
Pharmaceutical Applications
An imidazoquinoline used for the treatment of genital and perianal warts. While the mechanism of action is not precisely known, it is thought to induce interferon. It has no direct antiviral activity. The 5% cream applied three times a week for up to 16 weeks resulted in total wart clearance in 50% of patients, with a better response in women than in men. Local reactions are common and include erythema, erosion, excoriation and edema.
Biological Activity
Immunomodulator that displays antiviral and antitumor activity. Acts as a Toll-like receptor 7 (TLR-7) agonist; stimulates proinflammatory cytokine production and activates NF- κ B.
Biochem/physiol Actions
Imiquimod is a caspase 3 activator, which directly induces procaspase 3 cleavage to active caspase 3. Imiquimod induces apoptosis in vivo in basal cell carcinoma. Its anti-tumor activity is related to the induction of apoptosis. Imiquimod has anti-angiogenic, anti-inflammatory, anti-viral activities. Imiquimod also acts as an immune response modulator inducing the secretion of various cytokines and chemokines.
Mechanism of action
Imiquimod (Aldara) is an imidazoquinolone amine that is an immune responsemodifier.
Its exact mechanism is unknown in the topical treatment of HPV and molluscum
contagiosum but may be related to the immunomodulating effect of the drug. It is also
effective in the treatment of actinic keratoses and superficial basal cell carcinoma.
It induces production of a variety of cytokines and can enhance cell-mediated
cytolytic antiviral activity. It is a rapid and potent inducer of interferon-α,
interleukin?1 α and β, interleukin 6, interleukin 8, tumor necrosis factor-α,
granulocyte-macrophage colony-stimulating factor, and granulocyte colonystimulating
factor. Systemic absorption is minimal. It is generally well tolerated.
Adverse local reactions include erythema, erosion, excoriation, flaking, and edema
of the treatment sites.
Pharmacokinetics
Imiquimod is an immune response modifier that acts as a toll-like receptor 7 agonist. Imiquimod is commonly used topically to treat warts on the skin of the genital and anal areas. Imiquimod does not cure warts, and new warts may appear during treatment. Imiquimod does not fight the viruses that cause warts directly, however, it does help to relieve and control wart production. It is not used on warts inside the vagina, penis, or rectum. Imiquimod is also used to treat a skin condition of the face and scalp called actinic keratoses. Imiquimod can also be used to treat certain types of skin cancer called superficial basal cell carcinoma. Imiquimod is particularly useful on areas where surgery or other treatments may be difficult, complicated or otherwise undesirable, especially the face and lower legs.
Veterinary Drugs and Treatments
An immune response modifier, imiquimod may be useful in the treatment of a variety of topical conditions in animals. It is labeled for use
on humans as a treatment for genital or perianal warts, superficial basal cell carcinomas and actinic keratoses of the face and scalp. In dogs
and cats, imiquimod potentially may be of benefit in treating feline herpes virus dermatitis, actinic keratosis, squamous cell carcinoma
and Bowen’s disease, papillomas virus lesions, and localized solar dermatitis or solar carcinoma in situ. In horses, imiquimod has been
anecdotally used with success in treating sarcoids.
Imiquimod stimulates the patient’s own immune system to release a variety of cytokines including interferon-alpha and interleukin-
12. Imiquimod itself does not have in vitro activity against wart viruses, but stimulates monocytes and macrophages to release cytokines
that induce a regression in viral protein production.
Metabolism
Not Available
storage
+4°C
References
1) Hemmi?et al.?(2002),?Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway;?Nat. Immunol.?3?196 2) Stanley?et al.?(2002),?Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential;?Clin. Exp. Dermatol.?27?571 3) Schoen?et al.?(2006),?The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7- and TLR8-independent fashion;?J.Invest.Dermatol.?126?1338 4) Kan?et al.?(2012),?Imiquimod Suppresses Propagation of Herpes Simplex Virus 1 by Upregulation of Cystatin A via the Adenosine Receptor A1?Pathway.; J. Virol.?86?10338 5) Urosevic?et al.?(2004),?Imiquimod Treatment Induces Expression of Opioid Growth Factor Receptor; Clin. Cancer Res.?10?4959 6) Zagon?et al.?(2008),?Imiquimod Upregulates the Opioid Growth Factor Receptor to Inhibit Cell Proliferation Independent of Immune Function.; Exp. Biol. Med.(Maywood)?233?968
Properties of Imiquimod
| Melting point: | 292-294°C |
| Boiling point: | 456.7±48.0 °C(Predicted) |
| Density | 1.28±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C |
| solubility | DMSO: soluble |
| form | White solid |
| pka | 6.28±0.30(Predicted) |
| color | White |
| Water Solubility | Soluble in dimethyl sulfoxide and dimethyl formamide. Slightly soluble in water. Insoluble in ethanol. |
| λmax | 245nm(H2O/MeOH)(lit.) |
| Merck | 14,4922 |
| Stability: | Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 99011-02-6(CAS DataBase Reference) |
Safety information for Imiquimod
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Imiquimod
| InChIKey | DOUYETYNHWVLEO-UHFFFAOYSA-N |
Imiquimod manufacturer
Solara Active Pharma Sciences Ltd
Ralington Pharma
BDR Pharmaceuticals International Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![1-(2-METHYLPROPYL)-1H-IMIDAZO[4,5-C]QUINOLINE](https://img.chemicalbook.in/CAS/GIF/99010-24-9.gif)
![Imiquimod Related Compound B (25 mg) (1-Isobutyl-1H-imidazo[4,5-c]quinoline 5-oxide)](https://img.chemicalbook.in/CAS/20150408/GIF/99010-63-6.gif)
![Imiquimod Related Compound D (25 mg) (1-Propyl-1H-imidazo[4,5-c]quinolin-4-amine)](https://img.chemicalbook.in/CAS/20180808/GIF/853792-81-1.gif)
![[99010-09-0], C13H17N3,215.30](https://img.chemicalbook.in/CAS/GIF/935521-01-0.gif)
You may like
-
 Imiquimod 98%View Details
Imiquimod 98%View Details -
 99011-02-6 98%View Details
99011-02-6 98%View Details
99011-02-6 -
 Imiquimod 98% (HPLC) CAS 99011-02-6View Details
Imiquimod 98% (HPLC) CAS 99011-02-6View Details
99011-02-6 -
 Imiquimod CAS 99011-02-6View Details
Imiquimod CAS 99011-02-6View Details
99011-02-6 -
 Imiquimod 98%View Details
Imiquimod 98%View Details
99011-02-6 -
 99011-02-6 Imiquimod 98%View Details
99011-02-6 Imiquimod 98%View Details
99011-02-6 -
 Imiquimod 96% CAS 99011-02-6View Details
Imiquimod 96% CAS 99011-02-6View Details
99011-02-6 -
 Imiquimod CAS 99011-02-6View Details
Imiquimod CAS 99011-02-6View Details
99011-02-6