Imipenem-Cilastatin sodium hydrate
- CAS NO.:92309-29-0
- Empirical Formula: C28H43N5O9S2
- Molecular Weight: 657.8
- EINECS: 1592732-453-0
- SAFETY DATA SHEET (SDS)
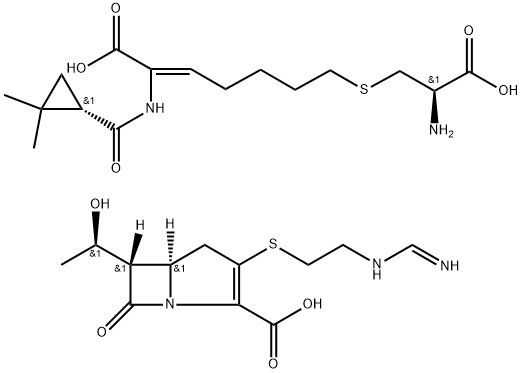
What is Imipenem-Cilastatin sodium hydrate?
Clinical Use
Antibacterial agent
Drug interactions
Potentially hazardous interactions with other drugs
Antiepileptics: reduced valproate concentration -
avoid.
Antivirals: convulsions reported with concomitant
administration of ganciclovir and valganciclovir.
Ciclosporin: variable reports of increase / no change
in ciclosporin levels, and of neurotoxicity.
Metabolism
When administered alone, imipenem is metabolised in the kidneys by dehydropeptidase-I, an enzyme in the brush border of the renal tubules, to inactive, nephrotoxic metabolites, with only about 5 to 40 or 45% of a dose excreted in the urine as unchanged active drug. Cilastin inhibits the metabolism of imipenem. When given with cilastatin about 70% of an intravenous dose of imipenem is recovered unchanged in the urine within 10 hours. Cilastatin is also excreted mainly in the urine, the majority as unchanged drug and about 12% as N-acetyl cilastatin. Less than 1% of imipenem is excreted via the bile in the faeces.
Safety information for Imipenem-Cilastatin sodium hydrate
Imipenem-Cilastatin sodium hydrate manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![(5R,6S)-3-[(Diphenoxyphosphinyl)oxy]-6-[(1R)-1-hydroxyethyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid (4-nitrophenyl)methyl ester](https://img.chemicalbook.in/CAS/GIF/75321-08-3.gif)
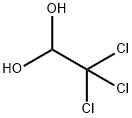
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8