Iminostilbene
Synonym(s):5H-Dibenz[b,f]azepine;Carbamazepine Related Compound B;Iminostilbene
- CAS NO.:256-96-2
- Empirical Formula: C14H11N
- Molecular Weight: 193.24
- MDL number: MFCD00005071
- EINECS: 205-970-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
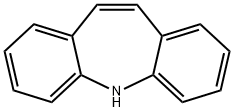
What is Iminostilbene?
Chemical properties
Yellow to orange-yellow fine powder
The Uses of Iminostilbene
Iminostilbene is mainly used as a pharmaceutical intermediate for the production of carbamazepine, Oxcarbazepine, and rhodium catalyst ligand. It is a metabolite of of carbamazepine which is used primarily in the treatment of epilepsy and neuropathic pain.
Definition
ChEBI: Iminostilbene is a mancude organic heterotricyclic parent that consists of a seven-membered nitrogen hetrocycle fused with two benzene rings. It has a role as a marine xenobiotic metabolite. It is a mancude organic heterotricyclic parent and a dibenzoazepine.
What are the applications of Application
Iminostilbene has good antioxidant effects. N-acetyl iminostilbene was synthesized by incubating iminostilbene (2.0020 g, 10.360 mmol) with acetic anhydride.
Preparation
iminostilbene synthesis: 10,11-Dihydro-5-dibenz(b,f)azepine [Iminodibenzyl, 494-19-9] as raw material was acylated by triphosgene, after bromination by bromine and dehydrobromination, reacted with sodium hydroxide in isopropanol to give Iminostilbene.
General Description
5H-Dibenz[b,f]azepine, a tricyclic amine with a seven-membered ring, is commonly known as iminostilbene. It is used as an intermediate or a starting material in the synthesis of many anticonvulsant drugs.It is also used as a starting material to prepare pharmacologically important dibenzoazepine-pyridazine derivatives and synthesize olefinic multidentate ligand, which is used to prepare Rh(I) complexes.
Biochem/physiol Actions
2-(Bromomethyl)naphthalene is a fluorescent alkyl bromide. It causes the esterification of free carboxyl groups formed at the surface of polyethylene terephthalate by enzyme hydrolysis. It acts as organic electrophile in the P4S10/acyloin reaction.
Properties of Iminostilbene
| Melting point: | 197 °C |
| Boiling point: | 221 °C(lit.) |
| Density | 1.290 g/mL at 20 °C(lit.) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | dioxane: 50 mg/mL, clear |
| form | powder |
| pka | 1.71±0.20(Predicted) |
| color | yellow to orange |
| Water Solubility | Soluble in ethyl acetate (25 mg/ml), water (partly), dioxane, chloroform, and DMSO. |
| BRN | 1343358 |
| CAS DataBase Reference | 256-96-2(CAS DataBase Reference) |
| NIST Chemistry Reference | o,o'-Iminostilbene(256-96-2) |
| EPA Substance Registry System | 5H-Dibenz[b,f]azepine (256-96-2) |
Safety information for Iminostilbene
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for Iminostilbene
Iminostilbene manufacturer
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran


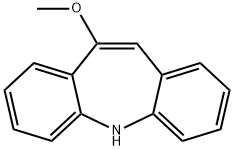
![Dibenz[b,f]azepine-5-carbonyl chloride](https://img.chemicalbook.in/CAS/GIF/33948-22-0.gif)
You may like
-
 Oxcarbazapine Impurity E 98%View Details
Oxcarbazapine Impurity E 98%View Details
256-96-2 -
 Iminostilbene 256-96-2 98%View Details
Iminostilbene 256-96-2 98%View Details
256-96-2 -
 Iminostilbene CAS 256-96-2View Details
Iminostilbene CAS 256-96-2View Details
256-96-2 -
 Iminostilbene 256-96-2 98View Details
Iminostilbene 256-96-2 98View Details
256-96-2 -
 256-96-2 Iminostilbene 99%View Details
256-96-2 Iminostilbene 99%View Details
256-96-2 -
 256-96-2 99%View Details
256-96-2 99%View Details
256-96-2 -
 Iminostilbene 98% CAS 256-96-2View Details
Iminostilbene 98% CAS 256-96-2View Details
256-96-2 -
 Carbamazepine Related Compound B CAS 256-96-2View Details
Carbamazepine Related Compound B CAS 256-96-2View Details
256-96-2
