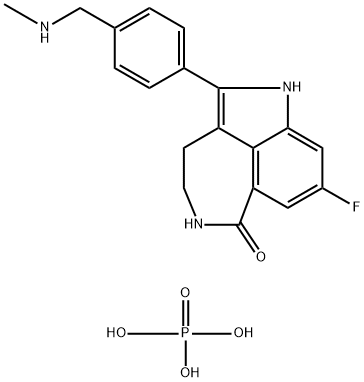
Imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one, 2-[4-[(dimethylamino)methyl]phenyl]-5,6-dihydro-
- CAS NO.:328543-09-5
- Empirical Formula: C19H20N4O
- Molecular Weight: 320.39
- MDL number: MFCD18385009
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
![Imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one, 2-[4-[(dimethylamino)methyl]phenyl]-5,6-dihydro- Structural](https://img.chemicalbook.in/CAS/GIF/328543-09-5.gif)
What is Imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one, 2-[4-[(dimethylamino)methyl]phenyl]-5,6-dihydro-?
Definition
ChEBI: LSM-1988 is a member of benzimidazoles.
Biological Activity
ag-14361 is a selective inhibitor of parp-1 with ki50 valueparp1 is a member of prap family and plays an important role in many cellular processes, such as dna repair, programmed cell death. it has been revealed that parp1 is abnormally expressed in a variety of cancers and many parp inhibitors have been developed as the anti-tumor drugs [1] [2] [3, 4].ag-14361 is a potent parp-1 inhibitor. when exposed hr and brca2-defective cells and parental cells to ag-14361, hr-defective cells were hypersensitive to the ag-14361 even at non-cytotoxic concentrations and lacking brca2 made the cells more sensitive to ag-14361 [5]. in human k562 cells, ag14361 treatment for 16 hours resulted in significant (~2-fold) potentiation of camptothecin-induced growth inhibition (gi50, 16 hours, camptothecin + ag14361 2.4 ± 0.1 nmol/l), cytotoxicity (lc50, camptothecin + ag14361 2.77 ± 0.55 nmol/l) and dna single-strand breaks via inhibiting parp-1 [1]. when tested with mmr-proficient (hct-ch3, a2780, and cp70-ch3) and mmr-deficient (hct116, cp70, and cp70-ch2) cells, mmr-proficient cells were more sensitivity to temozolomide compared with mmr-deficient cells after exposed to ag-14361 which inhibited parp1 activity [2].in mouse model xenografted with brca2-deficient and brca-2 proficient tumor cells, brca2 deficiency group had more response even completely regressed tumor compared with brca-2 proficient group when treated with ag-14361 [5].
References
[1]. smith, l.m., et al., the novel poly(adp-ribose) polymerase inhibitor, ag14361, sensitizes cells to topoisomerase i poisons by increasing the persistence of dna strand breaks. clin cancer res, 2005. 11(23): p. 8449-57.
[2]. curtin, n.j., et al., novel poly(adp-ribose) polymerase-1 inhibitor, ag14361, restores sensitivity to temozolomide in mismatch repair-deficient cells. clin cancer res, 2004. 10(3): p. 881-9.
[3]. calabrese, c.r., et al., anticancer chemosensitization and radiosensitization by the novel poly(adp-ribose) polymerase-1 inhibitor ag14361. j natl cancer inst, 2004. 96(1): p. 56-67.
[4]. veuger, s.j., et al., radiosensitization and dna repair inhibition by the combined use of novel inhibitors of dna-dependent protein kinase and poly(adp-ribose) polymerase-1. cancer res, 2003. 63(18): p. 6008-15.
[5]. kyle, s., et al., exploiting the achilles heel of cancer: the therapeutic potential of poly(adp-ribose) polymerase inhibitors in brca2-defective cancer. br j radiol, 2008. 81 spec no 1: p. s6-11.
Properties of Imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one, 2-[4-[(dimethylamino)methyl]phenyl]-5,6-dihydro-
| Density | 1.27 |
| storage temp. | Store at -20°C |
| solubility | insoluble in H2O; ≥10.14 mg/mL in EtOH with gentle warming and ultrasonic; ≥16 mg/mL in DMSO |
| form | solid |
| pka | 14.05±0.20(Predicted) |
| color | White to off-white |
Safety information for Imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one, 2-[4-[(dimethylamino)methyl]phenyl]-5,6-dihydro-
Computed Descriptors for Imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one, 2-[4-[(dimethylamino)methyl]phenyl]-5,6-dihydro-
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 AG14361 99% (HPLC) CAS 328543-09-5View Details
AG14361 99% (HPLC) CAS 328543-09-5View Details
328543-09-5 -
 AG14361 CAS 328543-09-5View Details
AG14361 CAS 328543-09-5View Details
328543-09-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8