Imazamox
Synonym(s):2-(4-Isopropyl-4-methyl-5-oxo-2-imidazolin-2-yl)-5-methoxymethylnicotinic acid;2-[4,5-Dihydro-4-methyl-4-(1-methylethyl)-5-oxo-1H-imidazol-2-yl]-5-(methoxymethyl)-3-pyridinecarboxylic acid;RAPTOR;Regulatory-associated protein of mTOR;RPTOR
- CAS NO.:114311-32-9
- Empirical Formula: C15H19N3O4
- Molecular Weight: 305.33
- MDL number: MFCD03427427
- EINECS: 601-305-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
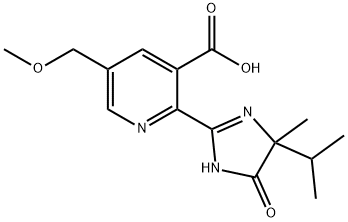
What is Imazamox?
Description
Imazamox is registered throughout the world for use in leguminous crops, including soybeans, alfalfa, and edible beans, as well as in imidazolinone-resistant crops (9). A nonionic surfactant or oil adjuvant is required for maximum activity. Imazamox is much more active when applied post-emergent to the weeds compared with pre-emergence application (9). One difference between imazamox and other imidazolinones is the much shorter interval needed before sensitive follow crops can be planted. This difference is due to the more rapid degradation of imazamox in the soil compared with other imidazolinones.
Chemical properties
Off-White Solid
The Uses of Imazamox
Imazamox is an imidazolinone based acetolactate synthase inhibitor that is utilized as a herbicide for weed control.
The Uses of Imazamox
Herbicide.
Definition
ChEBI: 2-(4-isopropyl-4-methyl-5-oxo-4,5-dihydro-1H-imidazol-2-yl)-5-(methoxymethyl)nicotinic acid is a pyridinemonocarboxylic acid that is nicotinic acid which is substituted substituted at position 5 by a methoxymethyl group and at position 2 by a 4,5-dihydro-1H-imidazol-2-yl group, that in turn is substituted by isopropyl, methyl, and oxo groups at positions 4, 4, and 5, respectively. It is a pyridinemonocarboxylic acid, an ether, an imidazolone and a member of imidazolines.
Pharmacology
Imazamox kills plants by inhibiting acetolactate synthase (ALS) (I50 = 1 μM), which is the first common enzyme in the biosynthesis of the branched chain amino acids, valine, leucine, and isoleucine. Imazamox is rapidly absorbed through the leaves of plants. Once it enters the plant, imazamox rapidly translocates to the growing points and growth ceases within 1 day after herbicide application, followed by chlorosis and then by necrosis of the growing points. Total plant death will occur within 2 to 3 weeks after treatment.
Metabolism
Plant Metabolism. The selectivity of imazamox is due to
differential rates and routes of metabolism in tolerant
crops versus susceptible weeds (10). The primary
metabolic route is hydroxylation followed by conjugation
to glucose. In imidazolinone-resistant crops, the primary
mechanism of selectivity is due to an altered acetolactate
synthase that is not inhibited by imazamox (11).
Animal Metabolism. Metabolism studies in the rat
showed that imazamox is rapidly excreted in the urine.
There was no accumulation of imazamox or any of its
derivatives in the liver, kidney, muscle, fat, or blood (9).
Toxicity evaluation
Imazamox has shown no mutagenic or genotoxic activity in the Ames assay, mammalian cell gene mutation assay, in vitro chromosome aberration assay, in vitro unscheduled DNA synthesis (URS) assay, or the in vivo dominant lethal assay inmale rats. The acute toxicity and effects on wildlife and soilmicroflora of imazamox are shown in Table 4. This herbicide also has a low potential for bioaccumulation in fish.
Properties of Imazamox
| Melting point: | 166-167°C |
| Density | 1.39 g/cm3 |
| storage temp. | 0-6°C |
| solubility | DMSO : 20 mg/mL (65.50 mM) |
| form | Solid |
| pka | pK1 2.3; pK2 3.3(at 25℃) |
| form | neat |
| color | White to off-white |
| InChI | InChI=1S/C15H19N3O4/c1-8(2)15(3)14(21)17-12(18-15)11-10(13(19)20)5-9(6-16-11)7-22-4/h5-6,8H,7H2,1-4H3,(H,19,20)(H,17,18,21) |
| CAS DataBase Reference | 114311-32-9(CAS DataBase Reference) |
| EPA Substance Registry System | Imazamox (114311-32-9) |
Safety information for Imazamox
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Imazamox
| InChIKey | NUPJIGQFXCQJBK-UHFFFAOYSA-N |
| SMILES | C1(C2NC(=O)C(C)(C(C)C)N=2)=NC=C(COC)C=C1C(O)=O |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 Imazamox 97% (HPLC) CAS 114311-32-9View Details
Imazamox 97% (HPLC) CAS 114311-32-9View Details
114311-32-9 -
 Imazamox CAS 114311-32-9View Details
Imazamox CAS 114311-32-9View Details
114311-32-9 -
 Imazamox CAS 114311-32-9View Details
Imazamox CAS 114311-32-9View Details
114311-32-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1