Hydroxylamine hydrochloride
Synonym(s):Hydroxylamine hydrochloride;Hydroxylammonium chloride;Hydroxylammonium Chloride, Hydroxyl Ammonium Chloride
- CAS NO.:5470-11-1
- Empirical Formula: NH2OH·HCl
- Molecular Weight: 69.49
- MDL number: MFCD00051089
- EINECS: 226-798-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:07
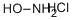
What is Hydroxylamine hydrochloride?
Description
Hydroxylamine hydrochloride is a reducing agent that is routinely used for the deacetylation of SATA to form free sulfhydryls (Figure 1), for cleavage of protein cross‐linkers that contain carbonyl groups (i.e. EGS) and for mutagenesis of plasmid DNA.
Hydroxylamine converts aldehydes and ketones (carbonyls) to their oxime derivative in weak bases, therefore cross‐linkers and other compounds with carbonyl groups are cleavable with Hydroxylamine hydrochloride.
SATA and SATP are modification reagents that add a sulfhydryl group to primary amines on biomolecules. The initial modification results in the addition of an acetyl‐protected sulfur enabling storage of the biomolecule. To generate a free sulfhydryl the biomolecule is treated with hydroxylamine to remove the protecting acetyl group (see figure).
EGS and sulfo‐EGS are homobifunctional, succinimidyl ester, amine reactive crosslinkers that are resistant to cleavage by denaturants used in SDS‐PAGE conditions, but may be cleaved with hydroxylamine.
Chemical properties
Hydroxylamine Hydrochloride(NH2OH.HCl) is an inorganic compound that is the hydrochloric acid salt of hydroxylamine. It is highly hygroscopic and decomposes when exposed to dampness above 151℃. This compound is primarily used as a reducing agent and imaging agent and is instrumental in the preparation of oximes in organic synthesis. It can convert aldehydes and ketones to oximes and acid chlorides to hydroxamic acids. However, This product is highly toxic and irritating to the skin.
Physical properties
It is a colorless monoclinic crystal that is hygroscopic and decomposes slowly in moist air. It has a density of 1.67 g/cm3 at a temperature of 17°C and melts at 151°C with decomposition. It is highly soluble in water, with a solubility of 84g/100g at 20°C, and is also soluble in lower alcohols and glycols. A 0.1 molar solution of this substance has a pH of 3.4.
Characteristics
Features of Hydroxylamine hydrochloride:
Quench amine-labeling or crosslinking reactions (e.g., with NHS esters)
Expose protected sulfhydryl groups on SATA-modified molecules
Cleave carbonyl-containing crosslinkers such as EGS and Sulfo-EGS
The Uses of Hydroxylamine hydrochloride
Hydroxylamine hydrochloride is a monomoamine oxidase inhibitor. It is used to prepare oximes and hydroxmic acids in organic synthesis. It acts as a copolymerization inhibitor. It can be used to remove bromine and polybromide from a solution during extraction of lignin from lignocellulosic biomass. It is key starting material for the preparation of pharmaceuticals and agrochemicals. It plays a vital role in rubber and plastic industries as an antioxidant, a vulcanization accelerator and a radical scavenger. It is also used as a color stabilizer and emulsion additive in color films.
What are the applications of Application
Hydroxylamine hydrochloride is an agent used as a catalyst and copolymerization inhibitor
Preparation
Hydroxylamine hydrochloride is prepared by electrolytic reduction of ammonium chloride. Or by the action of nitromethane with hydrochloric acid and water to obtain hydroxylamine hydrochloride.
Definition
ChEBI: Hydroxylamine hydrochloride is an organic molecular entity. It is a colorless inorganic compound (HONH2) used in organic synthesis and as a reducing agent, due to its ability to donate nitric oxide.
Biotechnological Applications
Hydroxylamine hydrochloride is a strong reducing agent that is useful in biochemical crosslinking applications, including the deacetylation of SATA and chemical cleavage of EGS and Sulfo-EGS. Hydroxylamine converts carbonyl compounds (aldehydes and ketones) to their oxime derivatives in the presence of a weak base. Therefore, crosslinkers and other compounds that contain a carbonyl within their structure are cleavable with hydroxylamine?HCl.
MAN0011201_HydroxylamineHCl_UG.pdf
General Description
Hydroxylamine hydrochloride appears as colorless or off-white crystalline solid. pH (0.1 molar aqueous solution) 3.4. pH (0.2 molar aqueous solution) 3.2. (NTP, 1992)
Air & Water Reactions
Hygroscopic. Sensitive to prolonged exposure to air. Water soluble. Reacts slowly with water.
Reactivity Profile
A powerful reducing agent. Reacts with bases and oxidizing agents.
Hazard
Toxic by ingestion, strong irritant to tissue.
Fire Hazard
Flash point data for Hydroxylamine hydrochloride are not available; however, Hydroxylamine hydrochloride is probably combustible.
Contact allergens
Hydroxylamine and its salts are used in various branches of industry, as reducing agents in color film developers or as reagents in laboratories.
Biochem/physiol Actions
MAO inhibitor; inhibits platelet aggregation.
Purification Methods
Crystallise the salt from aqueous75% ethanol or boiling methanol, and dry it under vacuum over CaSO4 or P2O5. It has also been dissolved in a minimum of water and saturated with HCl; after three such crystallisations, it is dried under a vacuum over CaCl2 and NaOH. Its solubility at 20o is 85% in H2O, 6% in EtOH and 12% in MeOH. [Hurd Inorg Synth I 87 1939, Semon in Org Synth Coll Vol I 318 1941.]
Properties of Hydroxylamine hydrochloride
| Melting point: | 155-157 °C (dec.)(lit.) |
| Density | 1.67 g/mL at 25 °C(lit.) |
| vapor pressure | 0.054 Pa (50 °C) |
| storage temp. | Store at +15°C to +25°C. |
| solubility | 470g/l |
| form | Liquid |
| color | White to off-white |
| PH | 2.5-3.5 (25℃, 50mg/mL in H2O) |
| Water Solubility | 560 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,4828 |
| BRN | 3539763 |
| Stability: | Substances to be avoided include strong oxidizing agents. Heating above 115 C may cause explosion; do not store above 65C. Moisture and air sensitive. |
| CAS DataBase Reference | 5470-11-1(CAS DataBase Reference) |
| EPA Substance Registry System | Hydroxylamine hydrochloride (5470-11-1) |
Safety information for Hydroxylamine hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H290:Corrosive to Metals H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H351:Carcinogenicity H373:Specific target organ toxicity, repeated exposure H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Hydroxylamine hydrochloride
| InChIKey | WTDHULULXKLSOZ-UHFFFAOYSA-N |
Hydroxylamine hydrochloride manufacturer
GVSK Pharma Private Limited
Indexim International
G C Int'L
Avenex Chemical Technologies LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




![2-[[(2-ethylphenyl)(2-hydroxyethyl)amino]methyl]-3,3-difluoro-Propanenitrile](https://img.chemicalbook.in/CAS/GIF/2647-14-5.gif)
You may like
-
 Hydroxylamine hydrochloride 99%View Details
Hydroxylamine hydrochloride 99%View Details -
 Hydroxylamine hydrochloride, For analysis ACS CAS 5470-11-1View Details
Hydroxylamine hydrochloride, For analysis ACS CAS 5470-11-1View Details
5470-11-1 -
 Hydroxylamine Hydrochloride, For analysis ACS Reagent CAS 5470-11-1View Details
Hydroxylamine Hydrochloride, For analysis ACS Reagent CAS 5470-11-1View Details
5470-11-1 -
 5470--11-1View Details
5470--11-1View Details
5470--11-1 -
 HYDROXYLAMINE HYDROCHLORIDE 99%View Details
HYDROXYLAMINE HYDROCHLORIDE 99%View Details -
 Hydroxylamine Hydrochloride CASView Details
Hydroxylamine Hydrochloride CASView Details -
 HYDROXYLAMINE HYDROCHLORIDE AR/ACS CASView Details
HYDROXYLAMINE HYDROCHLORIDE AR/ACS CASView Details -
 HYDROXYLAMINE HYDROCHLORIDE For Synthesis CASView Details
HYDROXYLAMINE HYDROCHLORIDE For Synthesis CASView Details
