Hydrazoic acid
- CAS NO.:7782-79-8
- Empirical Formula: HN3
- Molecular Weight: 43.02804
- EINECS: 231-965-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
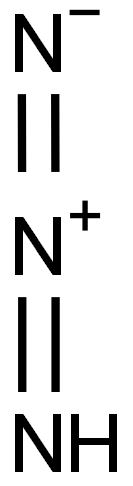
What is Hydrazoic acid?
Description
Hydrazoic acid or hydrogen azide is a dangerous explosion risk when shocked or heated. It is the gas-forming agent in many air bag systems in automobiles and escape chutes in airplanes.
Chemical properties
Colorless, volatile liquid; obnoxious odor.Soluble in water.
Physical properties
Colorless, volatile liquid; pungent disagreeable odor; density 1.09 g/mL;solidifies at -80°C; boils at 37°C; highly soluble in water; soluble in alkalies,alcohol and ether; pKa4.6 at 25°C.
The Uses of Hydrazoic acid
Hydrazoic acid is used in making heavymetal azides for detonators. It forms readilywhen sodium azide reacts with acid orhydrazine is mixed with nitrous acid.
The Uses of Hydrazoic acid
Industrially in preparation of heavy metal azides for shell detonators.
Definition
A colorless liquid with a nauseating smell. It is highly poisonous and explodes in the presence of oxygen and oxidizing agents. It can be made by distilling a mixture of sodium azide (NaN3) and a dilute acid. It is usually used as an aqueous solution. The salts of hydrazoic acid (azides), especially lead azide (Pb(N3)2), are used in detonators because of their ability to explode when given a mechanical shock.
Preparation
Hydrazoic acid is prepared by reacting sulfuric acid with sodium azide:
H2SO4 + NaN3 → HN3 + Na2SO4
or by treating hydrazine with nitrous acid:
N2H4 + HNO2 → HN3 + 2H2O
or by heating sodium amide with nitrous oxide:
NaNH2 + N2O → HN3 + NaOH
Production Methods
Hydrazoic acid is formed (1) by reaction of sodium nitrate with molten sodamide, (2) by reaction of nitrous oxide with molten sodamide, (3) by reaction of nitrous acid and hydrazinium ion (N2H5 + ), (4) by oxidation of hydrazinium salts, (5) by reaction of ethyl nitrite with NaOH solution and acidifying.
Reactions
Hydrazoic acid reacts (1) with metals, e.g., magnesium, aluminum, zinc, iron, to form azides or hydrazoates (or trinitrides), (2) with heavy metal salt solutions to form insoluble azides, e.g., silver azide AgN3, mercury(I) azide HgN3, lead azide PbN6. Silver, mercury(I), and copper(I) azides decompose in the light to form nitrogen plus the metal. (3) It reacts with NH4OH to form ammonium azide NH4·N3, (4) with hydrazine to form hydrazine azide N2H4·HN3, (5) with sodium hypochlorite plus acetic acid to form chlorazide ClN3, explosive, (6) with sodium amalgam to form NH3 with some hydrazine, (7) with potassium permanganate to form nitrogen and H2O.
Hazard
Dangerous explosion risk when shocked or heated. Strong irritant to eyes and mucous membranes.
Health Hazard
The acute toxicity of hydrazoic acidthrough inhalation and other routes of exposurehas been found to be high to very high.The symptoms and the intensity of poisoningare similar to sodium azide. It is, however,less toxic than hydrogen cyanide. Inhumans, inhalation of its vapors can produceirritation of eyes and respiratory tract, bronchitis,headache, dizziness, weakness, anddecreased blood pressure (Matheson 1983).Prolonged exposure to high concentrationscan result in collapse, convulsion, and death.An exposure to 1100 ppm for 1 hour waslethal to rats. Chronic exposure to a lowlevel of this compound in air may producehypotension.
Animals given intraperitoneal dosages ofhydrazoic acid showed the symptoms ofheavy breathing, convulsions, depression,and fall in blood pressure. It affected thecentral nervous system, but no damage wasobserved in the liver or kidney.
LD50 value, intraperitoneal (mice): 22 mg/kg.
Fire Hazard
In pure form or highly concentrated solution,
hydrazoic acid is a dangerous explosive compound.
It is unstable and sensitive to heat
and shock. The explosion hazard decreases
significantly with more dilute solutions.
It forms shock-sensitive metal azides
when react with metal salts, and fluorine
azide with fluorine (Lawless and Smith 1968)
and susceptible to form chlorine azide and
bromine azide with chlorine gas and bromine
vapor. All these products can explode violently
on impact. With carbon disulfide it
forms a violently explosive salt (Mellor
1946; NFPA 1997).
Potential Exposure
May be used in organic synthesis. Usedin making heavy metal azide detonators for explosives.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irri gate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contacts theskin, remove contaminated clothingand wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, includ-ing resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medi-cal attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
storage
Unstable and unpredictable explosive material.Prior to working with this chemical you should be trainedon its proper handling and storage. Store in tightly closedcontainers in a cool, well-venti lated area away from incom-patible materials listed above. Protect from heat and shock.
Incompatibilities
Explosive when dry; a highly sensitiveexplosive hazard when subject to shock or exposed to heat.Forms unstable compounds (heavy metal azides) with heavymetals. Forms explosive salts with carbon disulfide. Violentreaction with cadmium, copper, nickel, nitric acid, fluorine,heat, and shock.
Waste Disposal
Hydrazoic acid may be destroyed by convertingit to sodium azide. The latter isdecomposed with nitrous acid in a hood(National Research Council 1995). The followingmethod is used. It is diluted in waterto a strength below 5%; or its solution inorganic solvents that is immiscible in wateris shaken vigorously with water in a separatoryfunnel. The aqueous solution containinghydrazoic acid is neutralized with sodiumhydroxide and separated from any organiclayer. Sodium azide, so formed, is destroyedby reacting the aqueous solution with anexcess of sodium nitrite followed by 20%sulfuric acid until the solution is acidic. Thereaction is carried out in a three-necked flaskequipped with a stirrer, a dropping funnel,and a gas outlet line to vent out nitric oxide.The reaction mixture is flushed down thedrain.
Properties of Hydrazoic acid
| Melting point: | -80° |
| Boiling point: | bp 37° |
| Density | 1.092 |
| solubility | soluble in H2O |
| pka | 4.72(at 25℃) |
| form | colorless liquid |
| color | colorless liquid; explodes, explosive |
| Water Solubility | very soluble H2O [HAW93] |
| Exposure limits | Ceiling 0.1 ppm vapor (ACGIH). |
| Stability: | Unstable. Violently explosive in the concentrated or pure states. Readily forms explosive compounds with heavy metals. This is a dangerous and hard to handle substance which must not be prepared or handled by non-experts. |
| EPA Substance Registry System | Hydrazoic acid (7782-79-8) |
Safety information for Hydrazoic acid
Computed Descriptors for Hydrazoic acid
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1