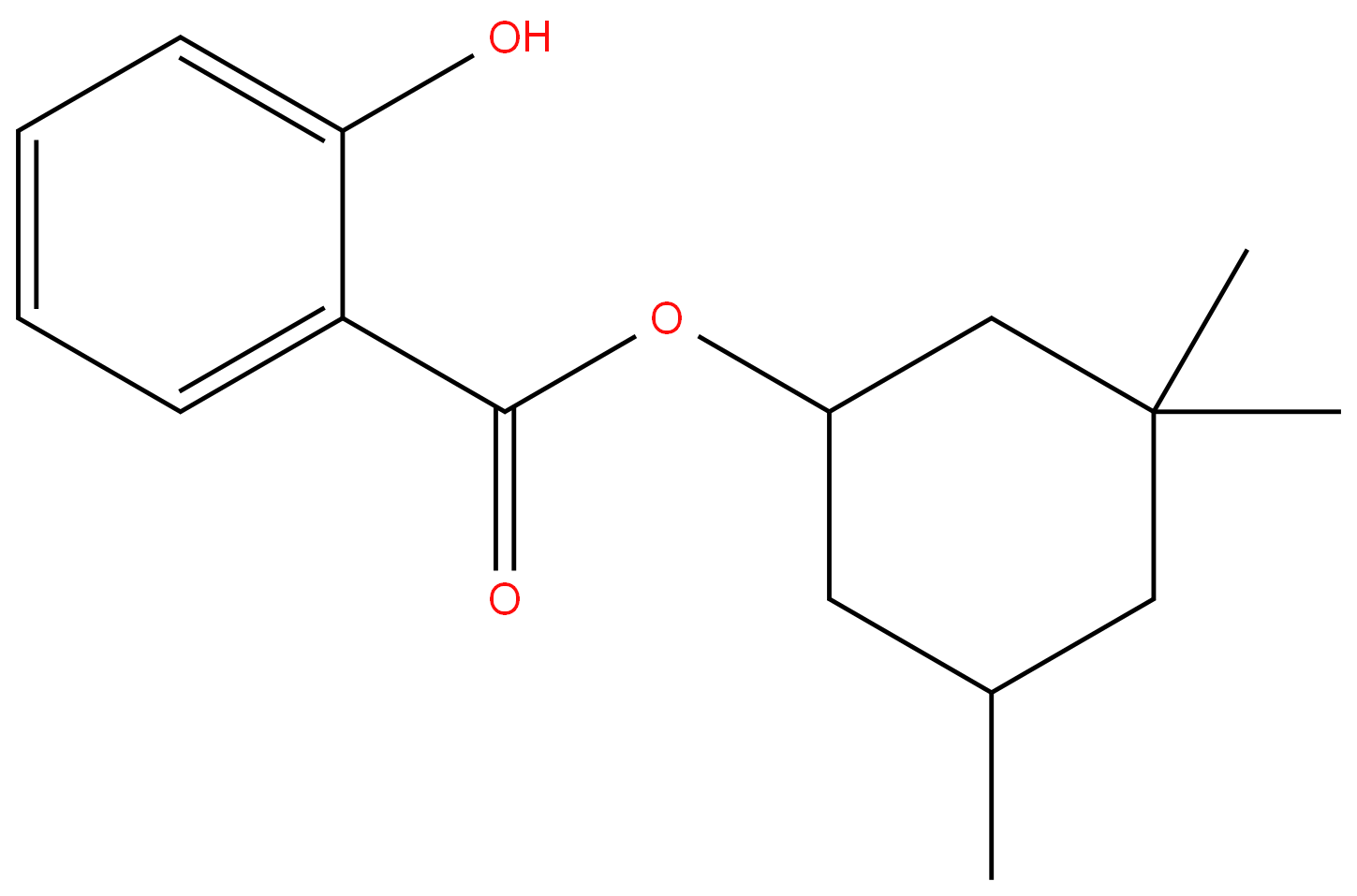
Homosalate
Synonym(s):2-Hydroxybenzoic acid 3,3,5-trimethylcyclohexyl ester;3,3,5-Trimethylcyclohexyl salicylate;Homomenthyl salicylate;Homosalate
- CAS NO.:118-56-9
- Empirical Formula: C16H22O3
- Molecular Weight: 262.34
- MDL number: MFCD00019377
- EINECS: 204-260-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-21 09:26:20
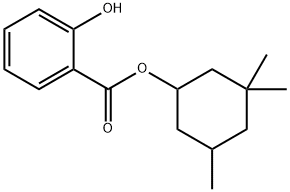
What is Homosalate?
Absorption
For local use only, no systemic absorption.
Toxicity
LD50: Not available.
Chemical properties
Liquid.
The Uses of Homosalate
homosalate is a chemical uVB absorber included in the FDA’s Category I Sunscreen Chemical list. Its approved usage level is 4 to 15 percent by the FDA and 10 percent by the european union’s Cosmetic Directive.
The Uses of Homosalate
UV screen, analgesic
The Uses of Homosalate
Homosalate used as a UV filter in various personal skin care formulations and cosmetics.
Indications
As ingredient in many sunscreen for protection against sunburn, skin aging and skin cancer.
Background
Homosalate is an organic compound that belongs to salicylates. It is an ester formed from salicylic acid and 3,3,5-trimethylcyclohexanol, a derivative of cyclohexanol. Salicylates prevent direct skin exposure to the sun’s harmful rays by absorbing ultraviolet (UV) light. Homosalate specifically absorbs short-wave UVB rays, which are associated with DNA damage and increased risk of skin cancer.
It is a common ingredient in many commercially available sunscreens. There are no reported adverse effects from homosalate.
Definition
A homolog of menthyl salicylate.
brand name
Eusolex (Rona Laboratories, Great Britain); Heliophan (Greeff).
General Description
Viscous or light yellow to slightly tan liquid or oil.
Air & Water Reactions
Homosalate will hydrolyze under basic conditions. . Insoluble in water.
Reactivity Profile
An ester and a phenol. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.
Fire Hazard
Homosalate is probably combustible.
Flammability and Explosibility
Non flammable
Pharmacokinetics
Acts as UV filters.
Metabolism
For local use only, no systemic absorption.
Properties of Homosalate
| Boiling point: | 161-165°C (12 torr) |
| Density | 1.05 |
| vapor pressure | 0.015Pa at 25℃ |
| refractive index | n20 1.516 to 1.518 |
| Flash point: | 169 - 173℃ |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Chloroform (Slightly), DMSO (Slightly) |
| form | neat |
| pka | 8.10±0.30(Predicted) |
| form | Liquid |
| color | Colourless |
| Odor | at 100.00?%. mild menthol |
| Water Solubility | <0.1 g/100 mL at 26 ºC |
| BRN | 2731604 |
| CAS DataBase Reference | 118-56-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Homosalate(118-56-9) |
| EPA Substance Registry System | 3,3,5-Trimethylcyclohexyl salicylate (118-56-9) |
Safety information for Homosalate
Computed Descriptors for Homosalate
Homosalate manufacturer
Ishwar Chemicals and Gases
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran
You may like
-
 118-56-9 HOMOSALATE 98%View Details
118-56-9 HOMOSALATE 98%View Details
118-56-9 -
 118-56-9 99%View Details
118-56-9 99%View Details
118-56-9 -
 Homosalate 98%View Details
Homosalate 98%View Details
118-56-9 -
 HOMOSALATE 99%View Details
HOMOSALATE 99%View Details
118-56-9 -
 3,3,5-Trimethylcyclohexyl Salicylate (cis- and trans- mixture) CAS 118-56-9View Details
3,3,5-Trimethylcyclohexyl Salicylate (cis- and trans- mixture) CAS 118-56-9View Details
118-56-9 -
 Homosalate 95% CAS 118-56-9View Details
Homosalate 95% CAS 118-56-9View Details
118-56-9 -
 Homosalate CAS 118-56-9View Details
Homosalate CAS 118-56-9View Details
118-56-9 -
 366789-02-8 Riveroxaban 98%View Details
366789-02-8 Riveroxaban 98%View Details
366789-02-8
