Hexafluoroacetone
- CAS NO.:684-16-2
- Empirical Formula: C3F6O
- Molecular Weight: 166.02
- MDL number: MFCD00000422
- EINECS: 211-676-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-14 15:18:26
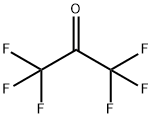
What is Hexafluoroacetone?
Description
Hexafluoroacetone is a colorless, nonflammablepoison gas. Musty odor. Shipped as a liquefied compressedgas. .Molecular weight= 163.00; Boiling point =- 28to - 26℃; FreezingMelting point= - 122℃; Relative vapordensity (air= 1)= 5.76; Vapor pressure = 5.8 atm at 25℃.Hazard Identification (based on NFPA-704 M Rating System):Health 3, Flammability 0, Reactivity 0. Reacts with water.
Chemical properties
Hexafluoroacetone is a colorless, nonflamma ble poison gas. Musty odor. Shipped as a liquefied com pressed gas.
Chemical properties
generally supplied as liquid under pressure
The Uses of Hexafluoroacetone
Hexafluoroacetone is a protecting and activatng reagent used in the synthesis of (S)-isoserine from (S)-malic acid. It is also an intermediate used to prepare hexafluorocarbinols as liver X receptor-α agonists.
The Uses of Hexafluoroacetone
Protecting and activating reagent in peptide chemistry; in synthesis of high performance fluoropolymers, pharmaceutical and agricultural chemicals; in 19F NMR. Solvent for polyamides, polyesters, polyacetals, polyols.
The Uses of Hexafluoroacetone
In the synthesis of polymer, pharmaceutical, and agricultural chemicals; solvent for polyamides, polyesters, and polyacetals; in the synthesis of hexafluoroisopropanol
Definition
ChEBI: A ketone that is acetone in which all the methyl hydrogens are replaced by fluoro groups.
Synthesis Reference(s)
Canadian Journal of Chemistry, 33, p. 453, 1955 DOI: 10.1139/v55-055
Organic Syntheses, Coll. Vol. 7, p. 251, 1990
General Description
Hexafluoroacetone is a colorless, toxic, and highly reactive gas. At ambient temperatures, Hexafluoroacetone is likely to generate a considerable amount of vapor. Hexafluoroacetone is an irritant to skin, eyes and mucous membranes and is toxic by ingestion, skin absorption, and inhalation. When heated to high temperatures Hexafluoroacetone emits toxic fluoride fumes. Prolonged exposure of the container to fire or intense heat may cause Hexafluoroacetone to violently rupture and rocket. Hexafluoroacetone is used in the production of other chemicals.
Air & Water Reactions
Hygroscopic (i.e., absorbs moisture from the air); reacts with moisture to form a highly acidic sesquihydrate. .
Reactivity Profile
Hexafluoroacetone is incompatible with the following: Water, acids [Note: Hygroscopic (i.e., absorbs moisture from the air); reacts with moisture to form a highly acidic sesquihydrate.] .
Hazard
Toxic by inhalation and skin absorption. Reacts vigorously with water and other substances, releasing considerable heat. Nonflammable.
Health Hazard
TOXIC; may be fatal if inhaled, ingested or absorbed through skin. Vapors are extremely irritating and corrosive. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
Fire Hazard
Some may burn but none ignite readily. Vapors from liquefied gas are initially heavier than air and spread along ground. Some of these materials may react violently with water. Cylinders exposed to fire may vent and release toxic and/or corrosive gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.
Potential Exposure
Hexafluoroacetone is used as a chemi cal intermediate. A gas at room temperature, it forms vari ous hydrates with water which are used as solvents for resins and polymers. Other derivatives are used to make water repellent coatings for textiles and also to produce polymers.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irri gate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled,remove from exposure,begin rescue breathing (using universal precautions, includ-ing resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medi-cal attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24- -48 h afterbreathing overexposure, as pulmonary edemamay bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a cortico-steroid spray. If frostbite has occurred, seek medical atten-tion immediately; do NOT rub the affected areas or flushthem with water. In order to prevent further tissue damage,do NOT attempt to remove frozen clothing from frostbittenareas. If frostbite has NOT occurred, immediately andthoroughly wash contaminated skin with soap and water.
storage
(1) Color Code- White stripe: Contact Hazard;Store separately; not compatible with materials in solidwhite category. (2) Color Code- Blue (poison gas): HealthHazard/Poison: Store in a secure poison location. Prior toworking with this chemical youshould be trained on itsproper handling and storage. Store in tightly closed contain-ers in a cool, well-ventilatedarea away from direct sunlight,water,heat, reducing agents, nitrates, and nitric acid.Procedures for the handling, use, and storage of cylindersshould be in compliance with OSHA 1910.101and1910.169, as with the recommendations of the CompressedGas A ssociation.
Shipping
UN2420 Hexafluoroacetone, Hazard Class: 2.3; Labels: 2.3-Poisonous gas, 8-Corrosive material, Inhalation Hazard Zone B. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the com pressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylin ders without the express written permission of the owner.
Purification Methods
Dehydrate hexafluoroacetone by passing the vapours over P2O5. Ethylene is removed by passing the dried vapours through a tube containing Pyrex glass wool moistened with conc H2SO4. Further purification is by low temperature distillation using Warde-Le Roy stills. Store it in the dark at -78o. [Holmes & Kutschke Trans Faraday Soc 58 333 1962, Beilstein 1 IV 3215.]
Incompatibilities
Reacts with water, oxidizers, strong acids. Hygroscopic (i.e., absorbs moisture from the air); reacts with moisture to form a highly acidic sesquihydrate and considerable heat.
Waste Disposal
Return refillable compressed gas cylinders to supplier. Nonrefillable cylinders should be disposed of in accordance with local, state and federal regulations. Allow remaining gas to vent slowly into atmosphere in an unconfined area or exhaust hood. Refillable-type cylinders should be returned to original sup plier with any valve caps and outlet plugs secured and valve protection caps in place.
Properties of Hexafluoroacetone
| Melting point: | −129 °C(lit.) |
| Boiling point: | −26 °C(lit.) |
| Density | 1,32 g/cm3 |
| vapor density | 1.65 (vs air) |
| vapor pressure | 4525 mm Hg ( 21.1 °C) |
| storage temp. | Refrigerator |
| solubility | Chloroform |
| form | Colorless gas, which reacts
vigorously with water to form hydrates |
| CAS DataBase Reference | 684-16-2(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Propanone, 1,1,1,3,3,3-hexafluoro-(684-16-2) |
| EPA Substance Registry System | Hexafluoroacetone (684-16-2) |
Safety information for Hexafluoroacetone
Computed Descriptors for Hexafluoroacetone
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4